Multifaceted peptide assisted one-pot synthesis of gold nanoparticles for plectin-1 targeted gemcitabine delivery in pancreatic cancer
- PMID: 28991294
- PMCID: PMC5859336
- DOI: 10.1039/c7nr03172f
Multifaceted peptide assisted one-pot synthesis of gold nanoparticles for plectin-1 targeted gemcitabine delivery in pancreatic cancer
Abstract
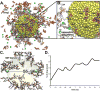
An astute modification of the plectin-1-targeting peptide KTLLPTP by introducing a C-terminal cysteine preceded by a tyrosine residue imparted a reducing property to the peptide. This novel property is then exploited to fabricate gold nanoparticles (GNP) via an in situ reduction of gold(iii) chloride in a one-pot, green synthesis. The modified peptide KTLLPTPYC also acts as a template to generate highly monodispersed, spherical GNPs with a narrow size distribution and improved stability. Plectin-1 is known to be aberrantly expressed in the surface of pancreatic ductal adenocarcinoma (PDAC) cells while showing cytoplasmic expression in normal cells. The synthesized GNPs are thus in situ surface modified with the peptides via the cysteine residue leaving the N-terminal KTLLPTP sequence free for targeting plectin-1. The visual molecular dynamics based simulations support the experimental observations like particle size, gemcitabine conjugation and architecture of the peptide-grafted nanoassembly. Additionally, GNPs conjugated to gemcitabine demonstrate significantly higher cytotoxicity in vitro in two established PDAC cell lines (AsPC-1 and PANC-1) and an admirable in vivo antitumor efficacy in a PANC-1 orthotopic xenograft model through selective uptake in PDAC tumor tissues. Altogether, this strategy represents a unique method for the fabrication of a GNP based targeted drug delivery platform using a multifaceted peptide that acts as reducing agent, template for GNP synthesis and targeting agent to display remarkable selectivity towards PDAC.
Conflict of interest statement
Conflicts of interest: The authors declare that they have no competing financial interests.
Figures





Similar articles
-
Plectin-1-targeted recognition for enhancing comprehensive therapy in pancreatic ductal adenocarcinoma.Nanoscale. 2024 Oct 10;16(39):18584-18596. doi: 10.1039/d4nr01587h. Nanoscale. 2024. PMID: 39291372
-
Nanomechanical Insight of Pancreatic Cancer Cell Membrane during Receptor Mediated Endocytosis of Targeted Gold Nanoparticles.ACS Appl Bio Mater. 2021 Jan 18;4(1):984-994. doi: 10.1021/acsabm.0c01443. Epub 2020 Dec 30. ACS Appl Bio Mater. 2021. PMID: 34913031 Free PMC article.
-
Targeted delivery of gemcitabine to pancreatic adenocarcinoma using cetuximab as a targeting agent.Cancer Res. 2008 Mar 15;68(6):1970-8. doi: 10.1158/0008-5472.CAN-07-6102. Cancer Res. 2008. PMID: 18339879
-
Self-Assembled Gemcitabine Prodrug Nanoparticles Show Enhanced Efficacy against Patient-Derived Pancreatic Ductal Adenocarcinoma.ACS Appl Mater Interfaces. 2020 Jan 22;12(3):3327-3340. doi: 10.1021/acsami.9b16209. Epub 2020 Jan 7. ACS Appl Mater Interfaces. 2020. PMID: 31872760
-
111In-Tetrameric Plectin-1 targeting peptide (4(βAKTLLPTP-GGS(PEG5000))KKK-111In-DOTA-βA-NH2).2011 Jan 28 [updated 2011 Apr 27]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2011 Jan 28 [updated 2011 Apr 27]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 21542555 Free Books & Documents. Review.
Cited by
-
Role of Functionalized Peptides in Nanomedicine for Effective Cancer Therapy.Biomedicines. 2024 Jan 16;12(1):202. doi: 10.3390/biomedicines12010202. Biomedicines. 2024. PMID: 38255307 Free PMC article. Review.
-
Dynamic alteration of poroelastic attributes as determinant membrane nanorheology for endocytosis of organ specific targeted gold nanoparticles.J Nanobiotechnology. 2022 Feb 8;20(1):74. doi: 10.1186/s12951-022-01276-1. J Nanobiotechnology. 2022. PMID: 35135558 Free PMC article.
-
Recent Advances in Well-Designed Therapeutic Nanosystems for the Pancreatic Ductal Adenocarcinoma Treatment Dilemma.Molecules. 2023 Feb 3;28(3):1506. doi: 10.3390/molecules28031506. Molecules. 2023. PMID: 36771172 Free PMC article. Review.
-
DNA-SWCNT Biosensors Allow Real-Time Monitoring of Therapeutic Responses in Pancreatic Ductal Adenocarcinoma.Cancer Res. 2019 Sep 1;79(17):4515-4523. doi: 10.1158/0008-5472.CAN-18-3337. Epub 2019 Jul 10. Cancer Res. 2019. PMID: 31292162 Free PMC article.
-
Plectin in Cancer: From Biomarker to Therapeutic Target.Cells. 2021 Aug 30;10(9):2246. doi: 10.3390/cells10092246. Cells. 2021. PMID: 34571895 Free PMC article. Review.
References
-
- Siegel RL, Miller KD, Jemal A. CA Cancer J Clin. 2017;67:7–30. - PubMed
-
- Goldstein D, El-Maraghi RH, Hammel P, Heinemann V, Kunzmann V, Sastre J, Scheithauer W, Siena S, Tabernero J, Teixeira L, Tortora G, Van Laethem JL, Young R, Penenberg DN, Lu B, Romano A, Von Hoff DD. J Natl Cancer Inst. 2015:107. - PubMed
-
- Papadatos-Pastos D, Thillai K, Rabbie R, Ross P, Sarker D. Expert Rev Anticancer Ther. 2014;14:1115–1125. - PubMed
-
- Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, Fouchardiere C de la, Bennouna J, Bachet JB, Khemissa-Akouz F, Pere-Verge D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M, U. Groupe Tumeurs Digestives of and P. Intergroup N Engl J Med. 2011;364:1817–1825. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

