Rapid changes in serum cytokines and chemokines in response to inactivated influenza vaccination
- PMID: 28991404
- PMCID: PMC5820426
- DOI: 10.1111/irv.12509
Rapid changes in serum cytokines and chemokines in response to inactivated influenza vaccination
Abstract
Background: The timing of host cytokine responses to influenza vaccination is poorly understood.
Objectives: We examined serum cytokine kinetics following inactivated trivalent influenza vaccine (TIV) to better understand potential relationships between markers of inflammation and TIV-related side effects.
Patients/methods: Twenty healthy adult subjects received TIV. Cytokines/chemokines were assessed in intervals from 3 hours to 14 days. Antibody titers were measured at baseline and Day 14.
Results: Serum cytokine responses to TIV were evident as early as 3 hours post-immunization. Compared to baseline, IFN-γ and IP-10 were significantly elevated 7 hours after TIV administration. Both remained elevated and peaked between 16 and 24 hours before returning to baseline by 44 hours post-vaccination. Although IL-8 levels were variable between subjects during the first 24 hours after TIV, by 44 hours, IL-8 was significantly lower compared to baseline. Interestingly, IL-8 levels remained significantly lower for up to 2 weeks after receiving TIV. Fifteen of 20 subjects reported mild adverse events. The one subject who reported moderate myalgias and injection site pain after vaccination displayed a distinctive, early cytokine response profile which included IL-6, IL-2, IL-8, IP-10, MCP-1, TNF-α, TARC, and MCP-4.
Conclusions: Serum cytokines changed rapidly following TIV and generally peaked at 24 hours. Trivalent influenza vaccine-induced reductions in IL-8 occurred later (44 hours) and were sustained for 2 weeks. An outlier response coincided with the only moderate side effects to the vaccine. These data suggest that early cytokine/chemokine responses may provide additional insight into the pathogenesis of adverse events and immune reactivity to vaccination.
Trial registration: ClinicalTrials.gov NCT01522248.
Keywords: chemokines; cytokines; inactivated; inactivated vaccine; influenza vaccine; symptoms.
© 2017 The Authors. Influenza and Other Respiratory Viruses Published by John Wiley & Sons Ltd.
Figures
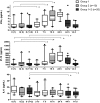
 ) had serum drawn at 0 h, 3 h, 7 h, 16 h, 24 h, 44 h, and 14 d post‐vaccination. Participants in group 2 (
) had serum drawn at 0 h, 3 h, 7 h, 16 h, 24 h, 44 h, and 14 d post‐vaccination. Participants in group 2 (  ) had serum drawn at 0 h, 16 h, 44 h, and 14 d post‐vaccination. Combined cytokine levels for groups 1 and 2 at 0 h, 44 h, and 14 d are also shown (
) had serum drawn at 0 h, 16 h, 44 h, and 14 d post‐vaccination. Combined cytokine levels for groups 1 and 2 at 0 h, 44 h, and 14 d are also shown (  ). The top and bottom of the box represent the 75th and 25th percentiles, respectively. The line near the middle of the box represents the median (50th percentile) and ends of the whiskers are drawn to the 10th and 90th percentiles. *P < .05, comparing pre‐ and post‐vaccination levels within each group. The Wilcoxon signed rank test was used to determine P‐values. Abbreviations: TIV, trivalent inactivated influenza vaccine; h, hours; d, days; n, number.
). The top and bottom of the box represent the 75th and 25th percentiles, respectively. The line near the middle of the box represents the median (50th percentile) and ends of the whiskers are drawn to the 10th and 90th percentiles. *P < .05, comparing pre‐ and post‐vaccination levels within each group. The Wilcoxon signed rank test was used to determine P‐values. Abbreviations: TIV, trivalent inactivated influenza vaccine; h, hours; d, days; n, number.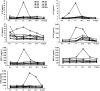
References
-
- Teijaro JR. The role of cytokine responses during influenza virus pathogenesis and potential therapeutic options. Curr Top Microbiol Immunol. 2015;386:3‐22. - PubMed
-
- Garcia‐Sastre A, Biron CA. Type 1 interferons and the virus‐host relationship: a lesson in detente. Science. 2006;312:879‐882. - PubMed
-
- Kobasa D, Jones SM, Shinya K, et al. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature. 2007;445:319‐323. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

