Synthetic Organic Electrochemical Methods Since 2000: On the Verge of a Renaissance
- PMID: 28991454
- PMCID: PMC5786875
- DOI: 10.1021/acs.chemrev.7b00397
Synthetic Organic Electrochemical Methods Since 2000: On the Verge of a Renaissance
Abstract
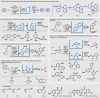
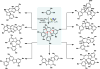
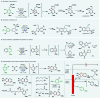
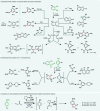
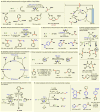
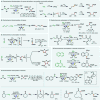
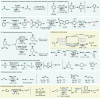
Electrochemistry represents one of the most intimate ways of interacting with molecules. This review discusses advances in synthetic organic electrochemistry since 2000. Enabling methods and synthetic applications are analyzed alongside innate advantages as well as future challenges of electroorganic chemistry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
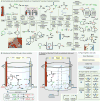


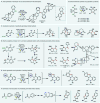
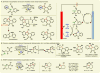
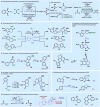






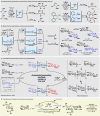
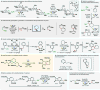

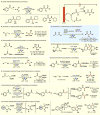

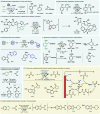




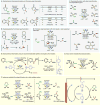

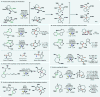

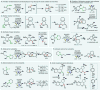
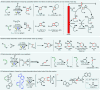




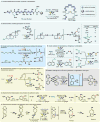

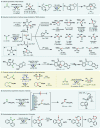

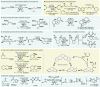

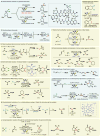





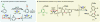
References
-
- Lund H. A Century of Organic Electrochemistry. J Electrochem Soc. 2002;149:S21–S33.
-
- Volta AGA. Nat Philos Chem Arts. 1800;4:179–187.
-
- Faraday M. Ann Phys Leipzig. 1834;47:438.
-
- Kolbe H. J Prakt Chem. 1847;41:138.
-
- Schoenbein ChF. Liebigs Ann Chem. 1845;54:164.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

