Functional and Biomimetic Materials for Engineering of the Three-Dimensional Cell Microenvironment
- PMID: 28991456
- PMCID: PMC6494624
- DOI: 10.1021/acs.chemrev.7b00094
Functional and Biomimetic Materials for Engineering of the Three-Dimensional Cell Microenvironment
Abstract
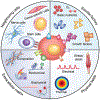
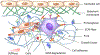
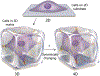
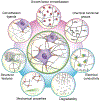
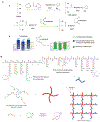
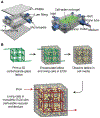
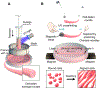
The cell microenvironment has emerged as a key determinant of cell behavior and function in development, physiology, and pathophysiology. The extracellular matrix (ECM) within the cell microenvironment serves not only as a structural foundation for cells but also as a source of three-dimensional (3D) biochemical and biophysical cues that trigger and regulate cell behaviors. Increasing evidence suggests that the 3D character of the microenvironment is required for development of many critical cell responses observed in vivo, fueling a surge in the development of functional and biomimetic materials for engineering the 3D cell microenvironment. Progress in the design of such materials has improved control of cell behaviors in 3D and advanced the fields of tissue regeneration, in vitro tissue models, large-scale cell differentiation, immunotherapy, and gene therapy. However, the field is still in its infancy, and discoveries about the nature of cell-microenvironment interactions continue to overturn much early progress in the field. Key challenges continue to be dissecting the roles of chemistry, structure, mechanics, and electrophysiology in the cell microenvironment, and understanding and harnessing the roles of periodicity and drift in these factors. This review encapsulates where recent advances appear to leave the ever-shifting state of the art, and it highlights areas in which substantial potential and uncertainty remain.
Figures



















References
-
- Scadden DT The stem-cell niche as an entity of action. Nature 2006, 441, 1075–1079. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

