Changes in Kidney Function Associated With Daily Tenofovir Disoproxil Fumarate/Emtricitabine for HIV Preexposure Prophylaxis Use in the United States Demonstration Project
- PMID: 28991887
- PMCID: PMC5762266
- DOI: 10.1097/QAI.0000000000001566
Changes in Kidney Function Associated With Daily Tenofovir Disoproxil Fumarate/Emtricitabine for HIV Preexposure Prophylaxis Use in the United States Demonstration Project
Abstract
Background: HIV preexposure prophylaxis (PrEP) using daily oral tenofovir-disoproxil-fumarate/emtricitabine (TDF/FTC) is effective for preventing HIV acquisition, but concerns remain about its potential kidney toxicity. This study examined kidney function in individuals using PrEP in real-world clinical settings.
Setting: Demonstration project in 2 sexually transmitted infection clinics and a community health center.
Methods: We evaluated kidney function among men who have sex with men and transgender women taking tenofovir-disoproxil-fumarate/emtricitabine PrEP for up to 48 weeks. Serum creatinine and urine dipstick for protein were obtained at 12-week intervals. Kidney function was estimated using creatinine clearance (CrCl) (Cockcroft-Gault) and estimated glomerular filtration rate (eGFR) (CKD-EPI).
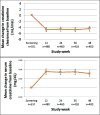
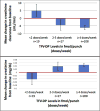
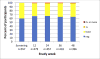
Results: From October 2012 to January 2014, we enrolled 557 participants (median age 33). Mean creatinine increased from baseline to week 12 by 0.03 mg/dL (4.6%) (P < 0.0001); mean CrCl decreased by 4.8 mL/min (3.0%) (P < 0.0001). These changes remained stable through week 48 (P = 0.81, P = 0.71 respectively). There were 75/478 (15.7%) participants who developed worsening proteinuria at week 12 compared with baseline (P < 0.0001), and this percent remained stable through week 48 (P = 0.73). Twenty-five participants (5.1%) developed new-onset eGFR <70 mL/min/1.73 m; independent predictors of this outcome were age ≥40 years (OR 3.79, 95% CI: 1.43 to 10.03) and baseline eGFR <90 mL/min/1.73 m (OR 9.59, 3.69-24.94).
Conclusions: In a demonstration setting, daily tenofovir-disoproxil-fumarate/emtricitabine PrEP leads to reduced CrCl and eGFR; however, these eGFR changes are based on very small changes in serum creatinine and seem to be nonprogressive after the first 12 weeks. Future studies are needed to understand the prognostic significance of these small changes.
Conflict of interest statement
The remaining authors have no conflicts of interest to declare.
Figures
Similar articles
-
Decline in Bone Mass With Tenofovir Disoproxil Fumarate/Emtricitabine Is Associated With Hormonal Changes in the Absence of Renal Impairment When Used by HIV-Uninfected Adolescent Boys and Young Men for HIV Preexposure Prophylaxis.Clin Infect Dis. 2017 Feb 1;64(3):317-325. doi: 10.1093/cid/ciw765. Epub 2016 Nov 15. Clin Infect Dis. 2017. PMID: 28013265 Free PMC article. Clinical Trial.
-
Kidney function and daily emtricitabine/tenofovir disoproxil fumarate pre-exposure prophylaxis against HIV: results from the real-life multicentric demonstrative project PrEP Brazil.AIDS Res Ther. 2022 Feb 24;19(1):12. doi: 10.1186/s12981-022-00437-4. AIDS Res Ther. 2022. PMID: 35209929 Free PMC article.
-
HIV-1 infection kinetics, drug resistance, and long-term safety of pre-exposure prophylaxis with emtricitabine plus tenofovir alafenamide (DISCOVER): week 144 open-label extension of a randomised, controlled, phase 3 trial.Lancet HIV. 2024 Aug;11(8):e508-e521. doi: 10.1016/S2352-3018(24)00130-9. Epub 2024 Jul 14. Lancet HIV. 2024. PMID: 39008999 Clinical Trial.
-
Should we fear resistance from tenofovir/emtricitabine preexposure prophylaxis?Curr Opin HIV AIDS. 2016 Jan;11(1):49-55. doi: 10.1097/COH.0000000000000209. Curr Opin HIV AIDS. 2016. PMID: 26633640 Free PMC article. Review.
-
Kidney function in tenofovir disoproxil fumarate-based oral pre-exposure prophylaxis users: a systematic review and meta-analysis of published literature and a multi-country meta-analysis of individual participant data.Lancet HIV. 2022 Apr;9(4):e242-e253. doi: 10.1016/S2352-3018(22)00004-2. Epub 2022 Mar 7. Lancet HIV. 2022. PMID: 35271825 Free PMC article.
Cited by
-
Translational Approach to Predicting the Efficacy of Maraviroc-Based Regimens as HIV Preexposure Prophylaxis.Antimicrob Agents Chemother. 2020 Jan 27;64(2):e01729-19. doi: 10.1128/AAC.01729-19. Print 2020 Jan 27. Antimicrob Agents Chemother. 2020. PMID: 31740561 Free PMC article.
-
Patient-Focused Selection of PrEP Medication for Individuals at Risk of HIV: A Narrative Review.Infect Dis Ther. 2021 Mar;10(1):165-186. doi: 10.1007/s40121-020-00384-5. Epub 2021 Feb 10. Infect Dis Ther. 2021. PMID: 33569743 Free PMC article. Review.
-
Review of Real-World Implementation Data on Emtricitabine-Tenofovir Disoproxil Fumarate as HIV Pre-exposure Prophylaxis in the United States.Pharmacotherapy. 2019 Apr;39(4):486-500. doi: 10.1002/phar.2240. Epub 2019 Apr 1. Pharmacotherapy. 2019. PMID: 30815960 Free PMC article. Review.
-
Human Vault Nanoparticle Targeted Delivery of Antiretroviral Drugs to Inhibit Human Immunodeficiency Virus Type 1 Infection.Bioconjug Chem. 2019 Aug 21;30(8):2216-2227. doi: 10.1021/acs.bioconjchem.9b00451. Epub 2019 Jul 16. Bioconjug Chem. 2019. PMID: 31265254 Free PMC article.
-
Lessons from a decade of voluntary medical male circumcision implementation and their application to HIV pre-exposure prophylaxis scale up.Int J STD AIDS. 2018 Dec;29(14):1432-1443. doi: 10.1177/0956462418787896. Epub 2018 Aug 16. Int J STD AIDS. 2018. PMID: 30114997 Free PMC article. Review.
References
-
- Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423–434. - PubMed
-
- Centers for Disease Control and Prevention. [Accessed June 16, 2017];Preexposure prophylaxis for the prevention of HIV in the United States - 2014 clinical practice guideline. Available at http://www.cdc.gov/hiv/pdf/prepguidelines2014.pdf.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

