Associations between an IgG3 polymorphism in the binding domain for FcRn, transplacental transfer of malaria-specific IgG3, and protection against Plasmodium falciparum malaria during infancy: A birth cohort study in Benin
- PMID: 28991911
- PMCID: PMC5633139
- DOI: 10.1371/journal.pmed.1002403
Associations between an IgG3 polymorphism in the binding domain for FcRn, transplacental transfer of malaria-specific IgG3, and protection against Plasmodium falciparum malaria during infancy: A birth cohort study in Benin
Abstract
Background: Transplacental transfer of maternal immunoglobulin G (IgG) to the fetus helps to protect against malaria and other infections in infancy. Recent studies have emphasized the important role of malaria-specific IgG3 in malaria immunity, and its transfer may reduce the risk of malaria in infancy. Human IgGs are actively transferred across the placenta by binding the neonatal Fc receptor (FcRn) expressed within the endosomes of the syncytiotrophoblastic membrane. Histidine at position 435 (H435) provides for optimal Fc-IgG binding. In contrast to other IgG subclasses, IgG3 is highly polymorphic and usually contains an arginine at position 435, which reduces its binding affinity to FcRn in vitro. The reduced binding to FcRn is associated with reduced transplacental transfer and reduced half-life of IgG3 in vivo. Some haplotypes of IgG3 have histidine at position 435. This study examines the hypotheses that the IgG3-H435 variant promotes increased transplacental transfer of malaria-specific antibodies and a prolonged IgG3 half-life in infants and that its presence correlates with protection against clinical malaria during infancy.
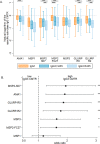
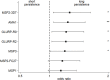
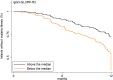
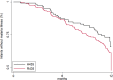
Methods and findings: In Benin, 497 mother-infant pairs were included in a longitudinal birth cohort. Both maternal and cord serum samples were assayed for levels of IgG1 and IgG3 specific for MSP119, MSP2 (both allelic families, 3D7 and FC27), MSP3, GLURP (both regions, R0 and R2), and AMA1 antigens of Plasmodium falciparum. Cord:maternal ratios were calculated. The maternal IgG3 gene was sequenced to identify the IgG3-H435 polymorphism. A multivariate logistic regression was used to examine the association between maternal IgG3-H435 polymorphism and transplacental transfer of IgG3, adjusting for hypergammaglobulinemia, maternal malaria, and infant malaria exposure. Twenty-four percent of Beninese women living in an area highly endemic for malaria had the IgG3-H435 allele (377 women homozygous for the IgG3-R435 allele, 117 women heterozygous for the IgG3-R/H alleles, and 3 women homozygous for the IgG3-H435 allele). Women with the IgG3-H435 allele had a 78% (95% CI 17%, 170%, p = 0.007) increased transplacental transfer of GLURP-R2 IgG3 compared to those without the IgG3-H435 allele. Furthermore, in infants born to mothers with the IgG3-H435 variant, a 28% longer IgG3 half-life was noted (95% CI 4%, 59%, p = 0.02) compared to infants born to mothers homozygous for the IgG3-R435 allele. Similar findings were observed for AMA1, MSP2-3D7, MSP3, GLURP-R0, and GLURP-R2 but not for MSP119 and MSP2-FC27. Infants born to women with IgG3-H435 had a 32% lower risk of symptomatic malaria during infancy (incidence rate ratio [IRR] = 0.68 [95% CI 0.51, 0.91], p = 0.01) compared to infants born to mothers homozygous for IgG3-R435. We did not find a lower risk of asymptomatic malaria in infants born to women with or without IgG3-H435. Limitations of the study were the inability to determine (i) the actual amount of IgG3-H435 relative to IgG-R435 in serum samples and (ii) the proportion of malaria-specific IgG produced by infants versus acquired from their mothers.
Conclusions: An arginine-to-histidine replacement at residue 435 in the binding domain of IgG3 to FcRn increases the transplacental transfer and half-life of malaria-specific IgG3 in young infants and is associated with reduced risk of clinical malaria during infancy. The IgG3-H435 allele may be under positive selection, given its relatively high frequency in malaria endemic areas.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Plasmodium falciparum merozoite surface antigen-specific cytophilic IgG and control of malaria infection in a Beninese birth cohort.Malar J. 2019 Jun 11;18(1):194. doi: 10.1186/s12936-019-2831-x. Malar J. 2019. PMID: 31185998 Free PMC article.
-
IgG and IgM responses to the Plasmodium falciparum asexual stage antigens reflect respectively protection against malaria during pregnancy and infanthood.Malar J. 2024 May 19;23(1):154. doi: 10.1186/s12936-024-04970-7. Malar J. 2024. PMID: 38764069 Free PMC article.
-
H435-containing immunoglobulin G3 allotypes are transported efficiently across the human placenta: implications for alloantibody-mediated diseases of the newborn.Transfusion. 2014 Mar;54(3):665-71. doi: 10.1111/trf.12334. Epub 2013 Jul 7. Transfusion. 2014. PMID: 23829325
-
Antibody Correlates of Protection from Clinical Plasmodium falciparum Malaria in an Area of Low and Unstable Malaria Transmission.Am J Trop Med Hyg. 2020 Dec;103(6):2174-2182. doi: 10.4269/ajtmh.18-0805. Epub 2020 Oct 27. Am J Trop Med Hyg. 2020. PMID: 33124533 Free PMC article. Review.
-
Relevance of the Materno-Fetal Interface for the Induction of Antigen-Specific Immune Tolerance.Front Immunol. 2020 May 14;11:810. doi: 10.3389/fimmu.2020.00810. eCollection 2020. Front Immunol. 2020. PMID: 32477339 Free PMC article. Review.
Cited by
-
The p.Arg435His Variation of IgG3 With High Affinity to FcRn Is Associated With Susceptibility for Pemphigus Vulgaris-Analysis of Four Different Ethnic Cohorts.Front Immunol. 2018 Aug 2;9:1788. doi: 10.3389/fimmu.2018.01788. eCollection 2018. Front Immunol. 2018. PMID: 30116249 Free PMC article.
-
Malaria and Early Life Immunity: Competence in Context.Front Immunol. 2021 Feb 19;12:634749. doi: 10.3389/fimmu.2021.634749. eCollection 2021. Front Immunol. 2021. PMID: 33679787 Free PMC article. Review.
-
Reduced FcRn-mediated transcytosis of IgG2 due to a missing Glycine in its lower hinge.Sci Rep. 2019 May 14;9(1):7363. doi: 10.1038/s41598-019-40731-2. Sci Rep. 2019. PMID: 31089170 Free PMC article.
-
Susceptibility to Plasmodium falciparum Malaria: Influence of Combined Polymorphisms of IgG3 Gm Allotypes and Fc Gamma Receptors IIA, IIIA, and IIIB.Front Immunol. 2020 Dec 23;11:608016. doi: 10.3389/fimmu.2020.608016. eCollection 2020. Front Immunol. 2020. PMID: 33424858 Free PMC article.
-
Impact of maternally derived antibodies to Plasmodium falciparum Schizont Egress Antigen-1 on the endogenous production of anti-PfSEA-1 in offspring.Vaccine. 2019 Aug 14;37(35):5044-5050. doi: 10.1016/j.vaccine.2019.06.084. Epub 2019 Jul 6. Vaccine. 2019. PMID: 31288996 Free PMC article.
References
-
- World Health Organization. World Malaria Report 2016. Geneva: World Health Organization; 2016. [cited 2017 Mar 17]. Available from: http://www.who.int/malaria/publications/world-malaria-report-2016/report....
-
- Murungi LM, Sondén K, Odera D, Oduor LB, Guleid F, Nkumama IN, et al. Cord blood IgG and the risk of severe Plasmodium falciparum malaria in the first year of life. Int J Parasitol. 2017;47(2–3):153–62. doi: 10.1016/j.ijpara.2016.09.005 - DOI - PMC - PubMed
-
- Abdullah S, Adazu K, Masanja H, Diallo D, Hodgson A, Ilboudo-Sanogo E, et al. Patterns of age-specific mortality in children in endemic areas of sub-Saharan Africa. Am J Trop Med Hyg. 2007;77:99–105. - PubMed
-
- Dobbs KR, Dent A. Plasmodium malaria and antimalarial antibodies in the first year of life. Parasitology. 2016;143:129–38. doi: 10.1017/S0031182015001626 - DOI - PMC - PubMed
-
- Stapleton NM, Andersen JT, Stemerding AM, Bjarnarson SP, Verheul RC, Gerritsen J, et al. Competition for FcRn-mediated transport gives rise to short half-life of human IgG3 and offers therapeutic potential. Nat Commun. 2011;2:599 doi: 10.1038/ncomms1608 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

