Can we further enrich autosomal dominant polycystic kidney disease clinical trials for rapidly progressive patients? Application of the PROPKD score in the TEMPO trial
- PMID: 28992127
- PMCID: PMC5888998
- DOI: 10.1093/ndt/gfx188
Can we further enrich autosomal dominant polycystic kidney disease clinical trials for rapidly progressive patients? Application of the PROPKD score in the TEMPO trial
Abstract
Background: The PROPKD score has been proposed to stratify the risk of progression to end-stage renal disease in autosomal dominant polycystic kidney disease (ADPKD) subjects. We aimed to assess its prognostic value in a genotyped subgroup of subjects from the Tolvaptan Phase 3 Efficacy and Safety Study in Autosomal Dominant Polycystic Kidney Disease (TEMPO3/4) trial.
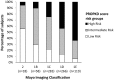
Methods: In the post hoc analysis, PKD1 and PKD2 were screened in 770 subjects and the PROPKD score was calculated in mutation-positive subjects (male: 1 point; hypertension <35 years: 2 points; first urologic event <35 years: 2 points; nontruncating PKD1 mutation: 2 points; truncating PKD1 mutation: 4 points). Subjects were classified into low-risk (LR; 0-3 points), intermediate-risk (IR; 4-6 points) and high-risk (HR; 7-9 points) groups.
Results: The PROPKD score was calculated in 749 subjects (LR = 132, IR = 344 and HR = 273); age was inversely related to risk (LR = 43.6 years, IR = 39.5 years, HR = 36.2 years; P < 0.001). Subjects from the HR group had significantly higher height-adjusted total kidney volume (TKV) and rates of TKV growth. While baseline renal function was similar across all risk groups, the rate of estimated glomerular filtration rate (eGFR) decline significantly increased from LR to HR in the placebo group. Tolvaptan treatment effectiveness to reduce TKV growth was similar in all three risk categories. While tolvaptan significantly slowed eGFR decline in the IR (tolvaptan = -2.34 versus placebo = -3.33 mL/min/1.73 m2/year; P = 0.008) and HR groups (tolvaptan = -2.74 versus placebo = -3.94 mL/min/1.73 m2/year; P = 0.002), there was no difference in the LR group (tolvaptan = -2.35 versus placebo = -2.50 mL/min/1.73 m2/year; P = 0.72). Excluding the LR subjects from the analysis improved the apparent treatment effect of tolvaptan on eGFR decline.
Conclusion: This study confirms the prognostic value of the PROPKD score and suggests that it could reduce costs and enhance endpoint sensitivity by enriching future study populations for rapidly progressing ADPKD subjects.
Figures


References
-
- Cornec-Le Gall EC-L, Audrézet M-P, Le Meur Y. et al. Genetics and pathogenesis of autosomal dominant polycystic kidney disease: twenty years on. Hum Mutat 2014; 35: 1393–1406 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

