Krüppel-like factors and vascular wall homeostasis
- PMID: 28992202
- PMCID: PMC5907833
- DOI: 10.1093/jmcb/mjx037
Krüppel-like factors and vascular wall homeostasis
Abstract
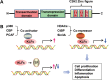
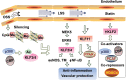
Cardiovascular diseases (CVDs) are major causes of death worldwide. Identification of promising targets for prevention and treatment of CVDs is paramount in the cardiovascular field. Numerous transcription factors regulate cellular function through modulation of specific genes and thereby are involved in the physiological and pathophysiological processes of CVDs. Although Krüppel-like factors (KLFs) have a similar protein structure with a conserved zinc finger domain, they possess distinct tissue and cell distribution patterns as well as biological functions. In the vascular system, KLF activities are regulated at both transcriptional and posttranscriptional levels. Growing in vitro, in vivo, and genetic epidemiology studies suggest that specific KLFs play important roles in vascular wall biology, which further affect vascular diseases. KLFs regulate various functional aspects such as cell growth, differentiation, activation, and development through controlling a whole cluster of functionally related genes and modulating various signaling pathways in response to pathological conditions. Therapeutic targeting of selective KLF family members may be desirable to achieve distinct treatment effects in the context of various vascular diseases. Further elucidation of the association of KLFs with human CVDs, their underlying molecular mechanisms, and precise protein structure studies will be essential to define KLFs as promising targets for therapeutic interventions in CVDs.
Keywords: atherosclerosis; drug development; endothelial cells; inflammation; shear stress; vascular injury; vascular smooth muscle cells.
© The Author (2017). Published by Oxford University Press on behalf of Journal of Molecular Cell Biology, IBCB, SIBS, CAS. All rights reserved.
Figures

References
-
- Bhattacharya R., SenBanerjee S., Lin Z., et al. (2005). Inhibition of vascular permeability factor/vascular endothelial growth factor-mediated angiogenesis by the Kruppel-like factor KLF2. J. Biol. Chem. 280, 28848–28851. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

