The plasma membrane H+-ATPase gene family in Solanum tuberosum L. Role of PHA1 in tuberization
- PMID: 28992210
- PMCID: PMC5853856
- DOI: 10.1093/jxb/erx284
The plasma membrane H+-ATPase gene family in Solanum tuberosum L. Role of PHA1 in tuberization
Abstract
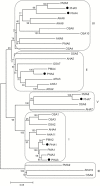
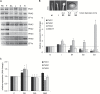
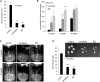
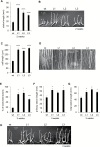
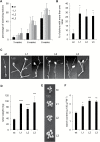
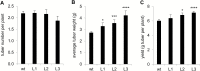
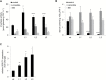
This study presents the characterization of the plasma membrane (PM) H+-ATPases in potato, focusing on their role in stolon and tuber development. Seven PM H+-ATPase genes were identified in the Solanum tuberosum genome, designated PHA1-PHA7. PHA genes show distinct expression patterns in different plant tissues and under different stress treatments. Application of PM H+-ATPase inhibitors arrests stolon growth, promotes tuber induction, and reduces tuber size, indicating that PM H+-ATPases are involved in tuberization, acting at different stages of the process. Transgenic potato plants overexpressing PHA1 were generated (PHA1-OE). At early developmental stages, PHA1-OE stolons elongate faster and show longer epidermal cells than wild-type stolons; this accelerated growth is accompanied by higher cell wall invertase activity, lower starch content, and higher expression of the sucrose-H+ symporter gene StSUT1. PHA1-OE stolons display an increased branching phenotype and develop larger tubers. PHA1-OE plants are taller and also present a highly branched phenotype. These results reveal a prominent role for PHA1 in plant growth and development. Regarding tuberization, PHA1 promotes stolon elongation at early stages, and tuber growth later on. PHA1 is involved in the sucrose-starch metabolism in stolons, possibly providing the driving force for sugar transporters to maintain the apoplastic sucrose transport during elongation.
Keywords: Branching; PHA1; PM H+-ATPase; plant growth; potato; stolon elongation; tuber growth; tuberization.
© The Author 2017. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Figures





References
-
- Ahn S, Im Y, Chung G, Cho B. 1999. Inducible expression of plasma membrane H+-ATPase in the roots of figleaf gourd plants under chilling root temperature. Physiologia Plantarum 106, 35–40.
-
- Albertson PL, Peters KF, Grof CPL. 2001. An improved method for the measurement of cell wall invertase activity in sugarcane tissue. Functional Plant Biology 28, 323–328.
-
- Appeldoorn NJG, de Bruijn SM, Koot-Gronsveld EAM, Visser RGF, Vreugdenhil D, van der Plas L. 1997. Developmental changes involved in conversion of sucrose to hexose-phosphate during early tuberization of potato. Planta 202, 220–226.
-
- Arango M, Gévaudant F, Oufattole M, Boutry M. 2003. The plasma membrane proton pump ATPase: the significance of gene subfamilies. Planta 216, 355–365. - PubMed
-
- Aue HL, Lecomte I, Gendraud M, Petel G. 1999. Change in plasma membrane ATPase activity during dormancy release of vegetative peach-tree buds. Physiologia Plantarum 106, 41–46.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

