Nitrogen Fixation via a Terminal Fe(IV) Nitride
- PMID: 28992418
- PMCID: PMC6021180
- DOI: 10.1021/jacs.7b09364
Nitrogen Fixation via a Terminal Fe(IV) Nitride
Abstract
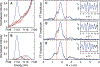
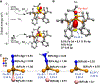
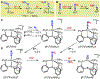
Terminal iron nitrides (Fe≡N) have been proposed as intermediates of (bio)catalytic nitrogen fixation, yet experimental evidence to support this hypothesis has been lacking. In particular, no prior synthetic examples of terminal Fe≡N species have been derived from N2. Here we show that a nitrogen-fixing Fe-N2 catalyst can be protonated to form a neutral Fe(NNH2) hydrazido(2-) intermediate, which, upon further protonation, heterolytically cleaves the N-N bond to release [FeIV≡N]+ and NH3. These observations provide direct evidence for the viability of a Chatt-type (distal) mechanism for Fe-mediated N2-to-NH3 conversion. The physical oxidation state range of the Fe complexes in this transformation is buffered by covalency with the ligand, a feature of possible relevance to catalyst design in synthetic and natural systems that facilitate multiproton/multielectron redox processes.
Figures

Similar articles
-
Characterization of the Earliest Intermediate of Fe-N2 Protonation: CW and Pulse EPR Detection of an Fe-NNH Species and Its Evolution to Fe-NNH2.J Am Chem Soc. 2019 May 22;141(20):8116-8127. doi: 10.1021/jacs.8b12082. Epub 2019 May 14. J Am Chem Soc. 2019. PMID: 31046258 Free PMC article.
-
N-H Bond Dissociation Enthalpies and Facile H Atom Transfers for Early Intermediates of Fe-N2 and Fe-CN Reductions.J Am Chem Soc. 2017 Mar 1;139(8):3161-3170. doi: 10.1021/jacs.6b12861. Epub 2017 Feb 17. J Am Chem Soc. 2017. PMID: 28140600 Free PMC article.
-
Characterization of an Fe≡N-NH2 Intermediate Relevant to Catalytic N2 Reduction to NH3.J Am Chem Soc. 2015 Jun 24;137(24):7803-7809. doi: 10.1021/jacs.5b03432. Epub 2015 Jun 10. J Am Chem Soc. 2015. PMID: 26000443 Free PMC article.
-
Catalytic N2-to-NH3 (or -N2H4) Conversion by Well-Defined Molecular Coordination Complexes.Chem Rev. 2020 Jun 24;120(12):5582-5636. doi: 10.1021/acs.chemrev.9b00638. Epub 2020 Apr 30. Chem Rev. 2020. PMID: 32352271 Free PMC article. Review.
-
Cleaving the n,n triple bond: the transformation of dinitrogen to ammonia by nitrogenases.Met Ions Life Sci. 2014;14:147-76. doi: 10.1007/978-94-017-9269-1_7. Met Ions Life Sci. 2014. PMID: 25416394 Review.
Cited by
-
Low-Valent Transition Metalate Anions in Synthesis, Small Molecule Activation, and Catalysis.Chem Rev. 2024 Feb 28;124(4):1323-1463. doi: 10.1021/acs.chemrev.3c00121. Epub 2024 Feb 14. Chem Rev. 2024. PMID: 38354371 Free PMC article. Review.
-
Light Alters the NH3 vs N2 H4 Product Profile in Iron-catalyzed Nitrogen Reduction via Dual Reactivity from an Iron Hydrazido (Fe=NNH2 ) Intermediate.Angew Chem Int Ed Engl. 2023 Feb 20;62(9):e202216693. doi: 10.1002/anie.202216693. Epub 2023 Jan 24. Angew Chem Int Ed Engl. 2023. PMID: 36592374 Free PMC article.
-
Catalytic Reduction of Cyanide to Ammonia and Methane at a Mononuclear Fe Site.J Am Chem Soc. 2024 Feb 28;146(8):5343-5354. doi: 10.1021/jacs.3c12395. Epub 2024 Feb 15. J Am Chem Soc. 2024. PMID: 38361429 Free PMC article.
-
Ammonia from dinitrogen at ambient conditions by organometallic catalysts.RSC Adv. 2022 Nov 23;12(52):33567-33583. doi: 10.1039/d2ra06156b. eCollection 2022 Nov 22. RSC Adv. 2022. PMID: 36505716 Free PMC article. Review.
-
Exploring the Limits of Dative Boratrane Bonding: Iron as a Strong Lewis Base in Low-Valent Non-Heme Iron-Nitrosyl Complexes.Inorg Chem. 2020 Oct 19;59(20):14967-14982. doi: 10.1021/acs.inorgchem.0c01686. Epub 2020 Sep 29. Inorg Chem. 2020. PMID: 32989992 Free PMC article.
References
-
- Hohenberger J; Ray K; Meyer K Nat. Commun 2012, 3, 720. - PubMed
-
- Chatt J; Dilworth JR; Richards RL Chem. Rev 1978, 78, 589.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

