Cell Sheet Morphogenesis: Dorsal Closure in Drosophila melanogaster as a Model System
- PMID: 28992442
- PMCID: PMC6524656
- DOI: 10.1146/annurev-cellbio-111315-125357
Cell Sheet Morphogenesis: Dorsal Closure in Drosophila melanogaster as a Model System
Abstract
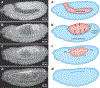
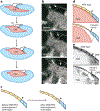
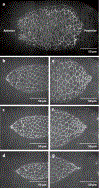
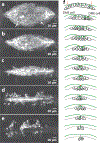
Dorsal closure is a key process during Drosophila morphogenesis that models cell sheet movements in chordates, including neural tube closure, palate formation, and wound healing. Closure occurs midway through embryogenesis and entails circumferential elongation of lateral epidermal cell sheets that close a dorsal hole filled with amnioserosa cells. Signaling pathways regulate the function of cellular structures and processes, including Actomyosin and microtubule cytoskeletons, cell-cell/cell-matrix adhesion complexes, and endocytosis/vesicle trafficking. These orchestrate complex shape changes and movements that entail interactions between five distinct cell types. Genetic and laser perturbation studies establish that closure is robust, resilient, and the consequence of redundancy that contributes to four distinct biophysical processes: contraction of the amnioserosa, contraction of supracellular Actomyosin cables, elongation (stretching?) of the lateral epidermis, and zipping together of two converging cell sheets. What triggers closure and what the emergent properties are that give rise to its extraordinary resilience and fidelity remain key, extant questions.
Keywords: Actomyosin; amnioserosa; biomechanics; ingression; morphogenetics; oscillation.
Figures

Similar articles
-
Identifying Key Genetic Regions for Cell Sheet Morphogenesis on Chromosome 2L Using a Drosophila Deficiency Screen in Dorsal Closure.G3 (Bethesda). 2020 Nov 5;10(11):4249-4269. doi: 10.1534/g3.120.401386. G3 (Bethesda). 2020. PMID: 32978263 Free PMC article.
-
Remodeling Tissue Interfaces and the Thermodynamics of Zipping during Dorsal Closure in Drosophila.Biophys J. 2015 Dec 1;109(11):2406-17. doi: 10.1016/j.bpj.2015.10.017. Biophys J. 2015. PMID: 26636951 Free PMC article.
-
Complete canthi removal reveals that forces from the amnioserosa alone are sufficient to drive dorsal closure in Drosophila.Mol Biol Cell. 2014 Nov 5;25(22):3552-68. doi: 10.1091/mbc.E14-07-1190. Epub 2014 Sep 24. Mol Biol Cell. 2014. PMID: 25253724 Free PMC article.
-
Mathematical models of dorsal closure.Prog Biophys Mol Biol. 2018 Sep;137:111-131. doi: 10.1016/j.pbiomolbio.2018.05.009. Epub 2018 May 29. Prog Biophys Mol Biol. 2018. PMID: 29852207 Free PMC article. Review.
-
Signaling pathways directing the movement and fusion of epithelial sheets: lessons from dorsal closure in Drosophila.Differentiation. 2002 Jun;70(4-5):181-203. doi: 10.1046/j.1432-0436.2002.700408.x. Differentiation. 2002. PMID: 12147138 Review.
Cited by
-
Identifying Key Genetic Regions for Cell Sheet Morphogenesis on Chromosome 2L Using a Drosophila Deficiency Screen in Dorsal Closure.G3 (Bethesda). 2020 Nov 5;10(11):4249-4269. doi: 10.1534/g3.120.401386. G3 (Bethesda). 2020. PMID: 32978263 Free PMC article.
-
Crosstalk between basal extracellular matrix adhesion and building of apical architecture during morphogenesis.Biol Open. 2021 Nov 15;10(11):bio058760. doi: 10.1242/bio.058760. Epub 2021 Nov 29. Biol Open. 2021. PMID: 34842274 Free PMC article. Review.
-
A minimal vertex model explains how the amnioserosa avoids fluidization during Drosophila dorsal closure.Proc Natl Acad Sci U S A. 2025 Jan 7;122(1):e2322732121. doi: 10.1073/pnas.2322732121. Epub 2024 Dec 30. Proc Natl Acad Sci U S A. 2025. PMID: 39793057 Free PMC article.
-
The Enigmas of Tissue Closure: Inspiration from Drosophila.Curr Issues Mol Biol. 2024 Aug 9;46(8):8710-8725. doi: 10.3390/cimb46080514. Curr Issues Mol Biol. 2024. PMID: 39194731 Free PMC article. Review.
-
Minimal vertex model explains how the amnioserosa avoids fluidization during Drosophila dorsal closure.ArXiv [Preprint]. 2023 Dec 20:arXiv:2312.12926v1. ArXiv. 2023. Update in: Proc Natl Acad Sci U S A. 2025 Jan 7;122(1):e2322732121. doi: 10.1073/pnas.2322732121. PMID: 38196754 Free PMC article. Updated. Preprint.
References
-
- Adachi-Yamada T, Fujimura-Kamada K, Nishida Y, Matsumoto K. 1999. Distortion of proximodistal information causes JNK-dependent apoptosis in Drosophila wing. Nature 400:166–69 - PubMed
-
- Almeida L, Bagnerini P, Habbal A, Noselli S, Serman F. 2011. A mathematical model for dorsal closure. J. Theor. Biol 268:105–19 - PubMed
-
- Bahri S, Wang S, Conder R, Choy J, Vlachos S, et al. 2010. The leading edge during dorsal closure as a model for epithelial plasticity: Pak is required for recruitment of the Scribble complex and septate junction formation. Development 137:2023–32 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

