A Theoretical Model of the Wnt Signaling Pathway in the Epithelial Mesenchymal Transition
- PMID: 28992816
- PMCID: PMC5634852
- DOI: 10.1186/s12976-017-0064-7
A Theoretical Model of the Wnt Signaling Pathway in the Epithelial Mesenchymal Transition
Abstract
Background: Following the formation of a primary carcinoma, neoplastic cells metastasize by undergoing the epithelial mesenchymal transition (EMT), which is triggered by cues from inflammatory and stromal cells in the microenvironment. EMT allows epithelial cells to lose their highly adhesive nature and instead adopt the spindle-like appearance, as well as the invasive and migratory behavior, of mesenchymal cells. We hypothesize that a bistable switch between the epithelial and mesenchymal phenotypes governs EMT, allowing the cell to maintain its mesenchymal phenotype even after it leaves the primary tumor microenvironment and EMT-inducing extracellular signal.
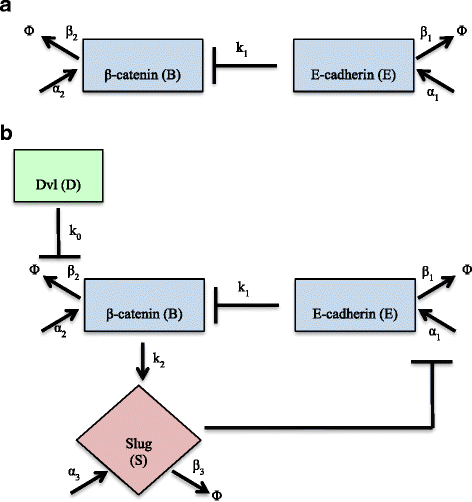
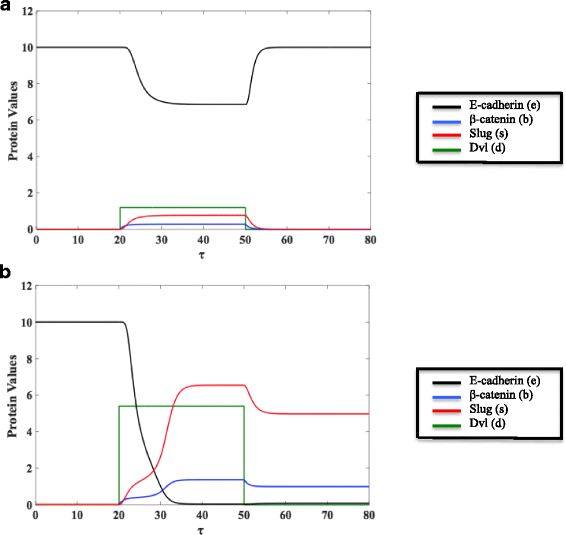
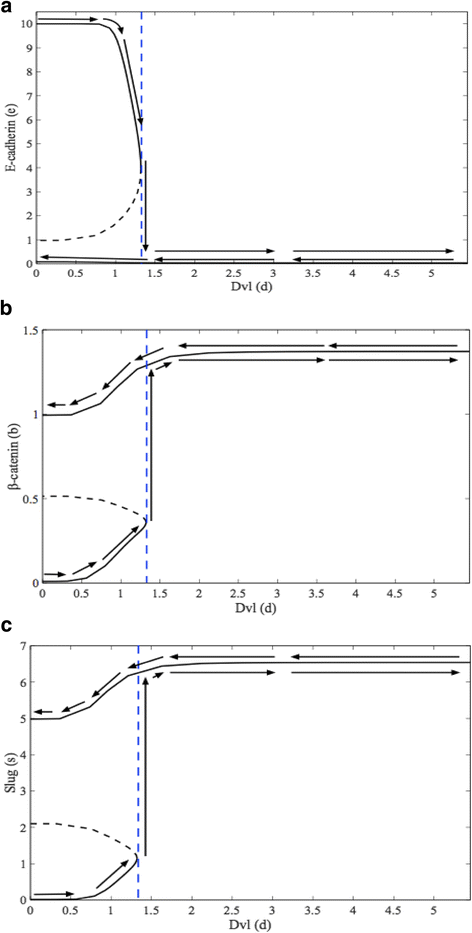
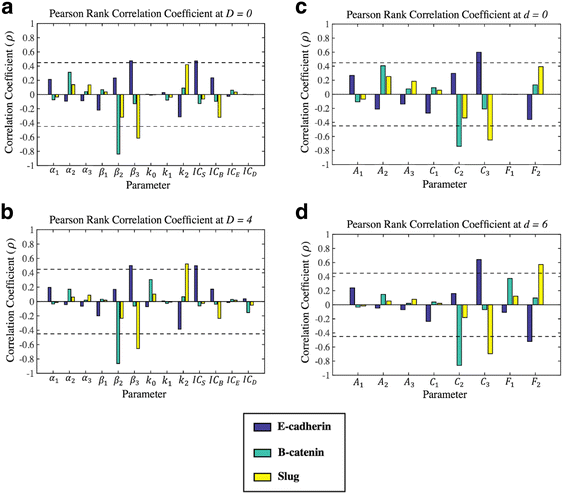
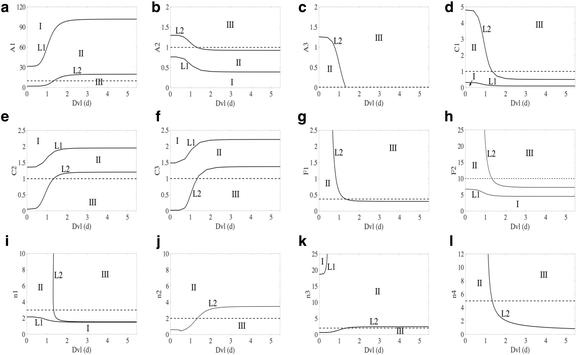
Results: This work presents a simple mathematical model of EMT, specifically the roles played by four key proteins in the Wnt signaling pathway: Dishevelled (Dvl), E-cadherin, β-catenin, and Slug. The model predicts that following activation of the Wnt pathway, an epithelial cell in the primary carcinoma must attain a threshold level of membrane-bound Dvl to convert to the mesenchymal-like phenotype and maintain that phenotype once it has migrated away from the primary tumor. Furthermore, sensitivity analysis of the model suggests that in both the epithelial and the mesenchymal states, the steady state behavior of E-cadherin and the transcription factor Slug are sensitive to changes in the degradation rate of Slug, while E-cadherin is also sensitive to the IC50 (half-maximal) concentration of Slug necessary to inhibit E-cadherin production. The steady state behavior of Slug exhibits sensitivity to changes in the rate at which it is induced by β-catenin upon activation of the Wnt pathway. In the presence of sufficient amount of Wnt ligand, E-cadherin levels are sensitive to the ratio of the rate of Slug activation via β-catenin to the IC50 concentration of Slug necessary to inhibit E-cadherin production.
Conclusions: The sensitivity of E-cadherin to the degradation rate of Slug, as well as the IC50 concentration of Slug necessary to inhibit E-cadherin production, shows how the adhesive nature of the cell depends on finely-tuned regulation of Slug. By highlighting the role of β-catenin in the activation of EMT and the relationship between E-cadherin and Slug, this model identifies critical parameters of therapeutic concern, such as the threshold level of Dvl necessary to inactivate the GSK-3β complex mediating β-catenin degradation, the rate at which β-catenin translocates to the nucleus, and the IC50 concentration of Slug needed to inhibit E-cadherin production.
Keywords: Bistable; Epithelial Mesenchymal Transition (EMT); Wnt.
Conflict of interest statement
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
3,5,4'-Trimethoxystilbene, a natural methoxylated analog of resveratrol, inhibits breast cancer cell invasiveness by downregulation of PI3K/Akt and Wnt/β-catenin signaling cascades and reversal of epithelial-mesenchymal transition.Toxicol Appl Pharmacol. 2013 Nov 1;272(3):746-56. doi: 10.1016/j.taap.2013.07.019. Epub 2013 Aug 3. Toxicol Appl Pharmacol. 2013. PMID: 23921149
-
Modeling the influence of cell-cell contact and TGF-β signaling on the epithelial mesenchymal transition in MCF7 breast carcinoma cells.J Theor Biol. 2022 Aug 7;546:111160. doi: 10.1016/j.jtbi.2022.111160. Epub 2022 May 17. J Theor Biol. 2022. PMID: 35594913
-
The transcription factor LEF-1 induces an epithelial-mesenchymal transition in MDCK cells independent of β-catenin.Biochem Biophys Res Commun. 2013 Dec 6;442(1-2):133-8. doi: 10.1016/j.bbrc.2013.11.031. Epub 2013 Nov 19. Biochem Biophys Res Commun. 2013. PMID: 24269234
-
Interplay between microRNAs and WNT/β-catenin signalling pathway regulates epithelial-mesenchymal transition in cancer.Eur J Cancer. 2015 Aug;51(12):1638-49. doi: 10.1016/j.ejca.2015.04.021. Epub 2015 May 26. Eur J Cancer. 2015. PMID: 26025765 Review.
-
Biomarkers of epithelial-mesenchymal transition: E-cadherin and beta-catenin in malignant transformation of oral lesions.Can J Dent Hyg. 2024 Jun 1;58(2):111-119. eCollection 2024 Jun. Can J Dent Hyg. 2024. PMID: 38974823 Free PMC article. Review.
Cited by
-
Neural control of body-plan axis in regenerating planaria.PLoS Comput Biol. 2019 Apr 16;15(4):e1006904. doi: 10.1371/journal.pcbi.1006904. eCollection 2019 Apr. PLoS Comput Biol. 2019. PMID: 30990801 Free PMC article.
-
Transcriptional regulators ensuring specific gene expression and decision-making at high TGFβ doses.Life Sci Alliance. 2024 Nov 14;8(1):e202402859. doi: 10.26508/lsa.202402859. Print 2025 Jan. Life Sci Alliance. 2024. PMID: 39542693 Free PMC article.
-
Tetraspanin 1 as a mediator of fibrosis inhibits EMT process and Smad2/3 and beta-catenin pathway in human pulmonary fibrosis.J Cell Mol Med. 2019 May;23(5):3583-3596. doi: 10.1111/jcmm.14258. Epub 2019 Mar 14. J Cell Mol Med. 2019. PMID: 30869194 Free PMC article.
-
Epithelial plasticity in COPD results in cellular unjamming due to an increase in polymerized actin.J Cell Sci. 2022 Feb 15;135(4):jcs258513. doi: 10.1242/jcs.258513. Epub 2022 Feb 24. J Cell Sci. 2022. PMID: 35118497 Free PMC article.
-
Epithelial-mesenchymal plasticity-engaging stemness in an interplay of phenotypes.Stem Cell Investig. 2019 Aug 20;6:25. doi: 10.21037/sci.2019.08.08. eCollection 2019. Stem Cell Investig. 2019. PMID: 31559312 Free PMC article. Review.
References
-
- Weinberg R. The biology of cancer: Garland science; 2013.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

