A Dynamic Stress Model Explains the Delayed Drug Effect in Artemisinin Treatment of Plasmodium falciparum
- PMID: 28993326
- PMCID: PMC5700357
- DOI: 10.1128/AAC.00618-17
A Dynamic Stress Model Explains the Delayed Drug Effect in Artemisinin Treatment of Plasmodium falciparum
Abstract
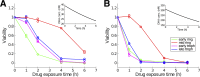
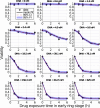
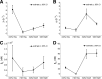
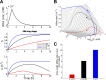
Artemisinin resistance constitutes a major threat to the continued success of control programs for malaria, particularly in light of developing resistance to partner drugs. Improving our understanding of how artemisinin-based drugs act and how resistance manifests is essential for the optimization of dosing regimens and the development of strategies to prolong the life span of current first-line treatment options. Recent short-drug-pulse in vitro experiments have shown that the parasite killing rate depends not only on drug concentration but also the exposure time, challenging the standard pharmacokinetic-pharmacodynamic (PK-PD) paradigm in which the killing rate depends only on drug concentration. Here, we introduce a dynamic stress model of parasite killing and show through application to 3D7 laboratory strain viability data that the inclusion of a time-dependent parasite stress response dramatically improves the model's explanatory power compared to that of a traditional PK-PD model. Our model demonstrates that the previously reported hypersensitivity of early-ring-stage parasites of the 3D7 strain to dihydroartemisinin compared to other parasite stages is due primarily to a faster development of stress rather than a higher maximum achievable killing rate. We also perform in vivo simulations using the dynamic stress model and demonstrate that the complex temporal features of artemisinin action observed in vitro have a significant impact on predictions for in vivo parasite clearance. Given the important role that PK-PD models play in the design of clinical trials for the evaluation of alternative drug dosing regimens, our novel model will contribute to the further development and improvement of antimalarial therapies.
Keywords: Plasmodium falciparum; artemisinin action; drug exposure time; dynamic model.
Copyright © 2017 Cao et al.
Figures

References
-
- World Health Organization. 2015. World malaria report, 2015. World Health Organization, Geneva, Switzerland.
-
- Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NP, Lindegardh N, Socheat D, White NJ. 2009. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 361:455–467. doi:10.1056/NEJMoa0808859. - DOI - PMC - PubMed
-
- Phyo AP, Nkhoma S, Stepniewska K, Ashley EA, Nair S, McGready R, ler Moo C, Al-Saai S, Dondorp AM, Lwin KM, Singhasivanon P, Day NP, White NJ, Anderson TJ, Nosten F. 2012. Emergence of artemisinin-resistant malaria on the western border of Thailand: a longitudinal study. Lancet 379:1960–1966. doi:10.1016/S0140-6736(12)60484-X. - DOI - PMC - PubMed
-
- Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois AC, Khim N, Kim S, Duru V, Bouchier C, Ma L, Lim P, Leang R, Duong S, Sreng S, Suon S, Chuor CM, Bout DM, Menard S, Rogers WO, Genton B, Fandeur T, Miotto O, Ringwald P, Le Bras J, Berry A, Barale JC, Fairhurst RM, Benoit-Vical F, Mercereau-Puijalon O, Menard D. 2014. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 505:50–55. doi:10.1038/nature12876. - DOI - PMC - PubMed
-
- Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, Sreng S, Anderson JM, Mao S, Sam B, Sopha C, Chuor CM, Nguon C, Sovannaroth S, Pukrittayakamee S, Jittamala P, Chotivanich K, Chutasmit K, Suchatsoonthorn C, Runcharoen R, Hien TT, Thuy-Nhien NT, Thanh NV, Phu NH, Htut Y, Han KT, Aye KH, Mokuolu OA, Olaosebikan RR, Folaranmi OO, Mayxay M, Khanthavong M, Hongvanthong B, Newton PN, Onyamboko MA, Fanello CI, Tshefu AK, Mishra N, Valecha N, Phyo AP, Nosten F, Yi P, Tripura R, Borrmann S, Bashraheil M, Peshu J, Faiz MA, Ghose A, Hossain MA, Samad R, Rahman MR, Hasan MM, Islam A, Miotto O, Amato R, MacInnis B, Stalker J, Kwiatkowski DP, Bozdech Z, Jeeyapant A, Cheah PY, Sakulthaew T, Chalk J, Intharabut B, Silamut K, Lee SJ, Vihokhern B, Kunasol C, Imwong M, Tarning J, Taylor WJ, Yeung S, Woodrow CJ, Flegg JA, Das D, Smith J, Venkatesan M, Plowe CV, Stepniewska K, Guerin PJ, Dondorp AM, Day NP, White NJ; Tracking Resistance to Artemisinin Collaboration (TRAC). 2014. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 371:411–423. doi:10.1056/NEJMoa1314981. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

