Evaluation of Pharmacokinetic/Pharmacodynamic Model-Based Optimized Combination Regimens against Multidrug-Resistant Pseudomonas aeruginosa in a Murine Thigh Infection Model by Using Humanized Dosing Schemes
- PMID: 28993331
- PMCID: PMC5700304
- DOI: 10.1128/AAC.01268-17
Evaluation of Pharmacokinetic/Pharmacodynamic Model-Based Optimized Combination Regimens against Multidrug-Resistant Pseudomonas aeruginosa in a Murine Thigh Infection Model by Using Humanized Dosing Schemes
Abstract
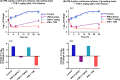
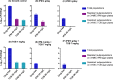
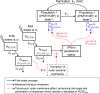
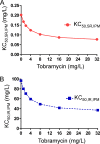
We previously optimized imipenem and tobramycin combination regimens against a double-resistant clinical Pseudomonas aeruginosa isolate by using in vitro infection models, mechanism-based pharmacokinetic/pharmacodynamic modeling (MBM), and Monte Carlo simulations. The current study aimed to evaluate these regimens in a neutropenic murine thigh infection model and to characterize the time course of bacterial killing and regrowth via MBM. We studied monotherapies and combinations of imipenem with tobramycin in vivo against the double-resistant clinical P. aeruginosa isolate by using humanized dosing schemes. Viable count profiles of total and resistant populations were quantified over 24 h. Tobramycin monotherapy (7 mg/kg every 24 h [q24h] as a 0.5-h infusion) was ineffective. Imipenem monotherapies (continuous infusion of 4 or 5 g/day with a 1-g loading dose) yielded 2.47 or 2.57 log10 CFU/thigh killing at 6 h. At 24 h, imipenem at 4 g/day led to regrowth up to the initial inoculum (4.79 ± 0.26 log10 CFU/thigh), whereas imipenem at 5 g/day displayed 1.75 log10 killing versus the initial inoculum. The combinations (i.e., imipenem at 4 or 5 g/day plus tobramycin) provided a clear benefit, with bacterial killing of ≥2.51 or ≥1.50 log10 CFU/thigh compared to the respective most active monotherapy at 24 h. No colonies were detected on 3×MIC agar plates for combinations, whereas increased resistance (at 3×MIC) emerged for monotherapies (except imipenem at 5 g/day). MBM suggested that tobramycin considerably enhanced the imipenem target site concentration up to 2.6-fold. The combination regimens, rationally optimized via a translational modeling approach, demonstrated substantially enhanced bacterial killing and suppression of regrowth in vivo against a double-resistant isolate and are therefore promising for future clinical evaluation.
Keywords: imipenem; mathematical modeling; neutropenic thigh infection model; population pharmacokinetics and pharmacodynamics; tobramycin.
Copyright © 2017 American Society for Microbiology.
Figures
References
-
- Boucher HW, Talbot GH, Benjamin DK Jr, Bradley J, Guidos RJ, Jones RN, Murray BE, Bonomo RA, Gilbert D. 2013. 10 × '20 progress–development of new drugs active against Gram-negative bacilli: an update from the Infectious Diseases Society of America. Clin Infect Dis 56:1685–1694. doi: 10.1093/cid/cit152. - DOI - PMC - PubMed
-
- Infectious Diseases Society of America (IDSA), Spellberg B, Blaser M, Guidos RJ, Boucher HW, Bradley JS, Eisenstein BI, Gerding D, Lynfield R, Reller LB, Rex J, Schwartz D, Septimus E, Tenover FC, Gilbert DN. 2011. Combating antimicrobial resistance: policy recommendations to save lives. Clin Infect Dis 52(Suppl 5):S397–S428. doi: 10.1093/cid/cir153. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

