The Electron Transport Chain Sensitizes Staphylococcus aureus and Enterococcus faecalis to the Oxidative Burst
- PMID: 28993457
- PMCID: PMC5695107
- DOI: 10.1128/IAI.00659-17
The Electron Transport Chain Sensitizes Staphylococcus aureus and Enterococcus faecalis to the Oxidative Burst
Abstract
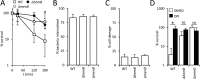
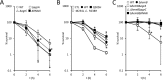
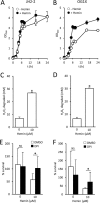
Small-colony variants (SCVs) of Staphylococcus aureus typically lack a functional electron transport chain and cannot produce virulence factors such as leukocidins, hemolysins, or the antioxidant staphyloxanthin. Despite this, SCVs are associated with persistent infections of the bloodstream, bones, and prosthetic devices. The survival of SCVs in the host has been ascribed to intracellular residency, biofilm formation, and resistance to antibiotics. However, the ability of SCVs to resist host defenses is largely uncharacterized. To address this, we measured the survival of wild-type and SCV S. aureus in whole human blood, which contains high numbers of neutrophils, the key defense against staphylococcal infection. Despite the loss of leukocidin production and staphyloxanthin biosynthesis, SCVs defective for heme or menaquinone biosynthesis were significantly more resistant to the oxidative burst than wild-type bacteria in human blood or the presence of purified neutrophils. Supplementation of the culture medium of the heme-auxotrophic SCV with heme, but not iron, restored growth, hemolysin and staphyloxanthin production, and sensitivity to the oxidative burst. Since Enterococcus faecalis is a natural heme auxotroph and cause of bloodstream infection, we explored whether restoration of the electron transport chain in this organism also affected survival in blood. Incubation of E. faecalis with heme increased growth and restored catalase activity but resulted in decreased survival in human blood via increased sensitivity to the oxidative burst. Therefore, the lack of functional electron transport chains in SCV S. aureus and wild-type E. faecalis results in reduced growth rate but provides resistance to a key immune defense mechanism.
Keywords: Enterococcus faecalis; Staphylococcus aureus; bacteremia; enterococcus; neutrophil; oxidative burst; small-colony variant.
Copyright © 2017 American Society for Microbiology.
Figures

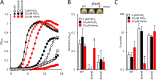
Similar articles
-
Heme-Dependent Siderophore Utilization Promotes Iron-Restricted Growth of the Staphylococcus aureus hemB Small-Colony Variant.J Bacteriol. 2021 Nov 19;203(24):e0045821. doi: 10.1128/JB.00458-21. Epub 2021 Oct 4. J Bacteriol. 2021. PMID: 34606375 Free PMC article.
-
The de novo Purine Biosynthesis Pathway Is the Only Commonly Regulated Cellular Pathway during Biofilm Formation in TSB-Based Medium in Staphylococcus aureus and Enterococcus faecalis.Microbiol Spectr. 2021 Dec 22;9(3):e0080421. doi: 10.1128/Spectrum.00804-21. Epub 2021 Dec 22. Microbiol Spectr. 2021. PMID: 34935415 Free PMC article.
-
Staphylococcus aureus Small Colony Variants (SCVs): a road map for the metabolic pathways involved in persistent infections.Front Cell Infect Microbiol. 2014 Jul 28;4:99. doi: 10.3389/fcimb.2014.00099. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 25120957 Free PMC article. Review.
-
Extracellular and intracellular bactericidal activities of XF-70 against small-colony variant hemB mutants of meticillin-susceptible and meticillin-resistant Staphylococcus aureus.Int J Antimicrob Agents. 2011 Jun;37(6):576-9. doi: 10.1016/j.ijantimicag.2011.01.015. Epub 2011 Mar 16. Int J Antimicrob Agents. 2011. PMID: 21414759
-
The small colony variant (SCV) concept -- the role of staphylococcal SCVs in persistent infections.Injury. 2006 May;37 Suppl 2:S26-33. doi: 10.1016/j.injury.2006.04.006. Injury. 2006. PMID: 16651068 Review.
Cited by
-
Metabolism, ATP production and biofilm generation by Staphylococcus epidermidis in either respiratory or fermentative conditions.AMB Express. 2020 Feb 11;10(1):31. doi: 10.1186/s13568-020-00966-z. AMB Express. 2020. PMID: 32048056 Free PMC article.
-
Staphylococcus aureus suppresses the pentose phosphate pathway in human neutrophils via the adenosine receptor A2aR to enhance intracellular survival.mBio. 2024 Jan 16;15(1):e0257123. doi: 10.1128/mbio.02571-23. Epub 2023 Dec 18. mBio. 2024. PMID: 38108639 Free PMC article.
-
RexAB Promotes the Survival of Staphylococcus aureus Exposed to Multiple Classes of Antibiotics.Antimicrob Agents Chemother. 2021 Sep 17;65(10):e0059421. doi: 10.1128/AAC.00594-21. Epub 2021 Jul 26. Antimicrob Agents Chemother. 2021. PMID: 34310219 Free PMC article.
-
Borosilicate bioactive glass synergizing low-dose antibiotic loaded implants to combat bacteria through ATP disruption and oxidative stress to sequentially achieve osseointegration.Bioact Mater. 2024 Oct 18;44:184-204. doi: 10.1016/j.bioactmat.2024.10.009. eCollection 2025 Feb. Bioact Mater. 2024. PMID: 39502840 Free PMC article.
-
Liberation of host heme by Clostridioides difficile-mediated damage enhances Enterococcus faecalis fitness during infection.mBio. 2024 Jan 16;15(1):e0165623. doi: 10.1128/mbio.01656-23. Epub 2023 Dec 11. mBio. 2024. PMID: 38078767 Free PMC article.
References
-
- Buvelot H, Posfay-Barbe KM, Linder P, Schrenzel J, Krause KH. 2017. Staphylococcus aureus, phagocyte NADPH oxidase and chronic granulomatous disease. FEMS Microbiol Rev 41:139–157. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

