Acquired Protective Immunity in Atlantic Salmon Salmo salar against the Myxozoan Kudoa thyrsites Involves Induction of MHIIβ+ CD83+ Antigen-Presenting Cells
- PMID: 28993459
- PMCID: PMC5736826
- DOI: 10.1128/IAI.00556-17
Acquired Protective Immunity in Atlantic Salmon Salmo salar against the Myxozoan Kudoa thyrsites Involves Induction of MHIIβ+ CD83+ Antigen-Presenting Cells
Abstract
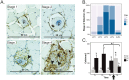
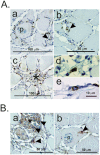
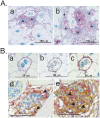
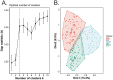
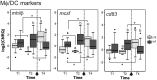
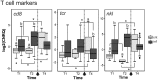
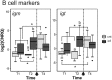
The histozoic myxozoan parasite Kudoa thyrsites causes postmortem myoliquefaction and is responsible for economic losses to salmon aquaculture in the Pacific Northwest. Despite its importance, little is known about the host-parasite relationship, including the host response to infection. The present work sought to characterize the immune response in Atlantic salmon during infection, recovery, and reexposure to K. thyrsites After exposure to infective seawater, infected and uninfected smolts were sampled three times over 4,275 degree-days. Histological analysis revealed infection severity decreased over time in exposed fish, while in controls there was no evidence of infection. Following a secondary exposure of all fish, severity of infection in the controls was similar to that measured in exposed fish at the first sampling time but was significantly reduced in reexposed fish, suggesting the acquisition of protective immunity. Using immunohistochemistry, we detected a population of MHIIβ+ cells in infected muscle that followed a pattern of abundance concordant with parasite prevalence. Infiltration of these cells into infected myocytes preceded destruction of the plasmodium and dissemination of myxospores. Dual labeling indicated a majority of these cells were CD83+/MHIIβ+ Using reverse transcription-quantitative PCR, we detected significant induction of cellular effectors, including macrophage/dendritic cells (mhii/cd83/mcsf), B cells (igm/igt), and cytotoxic T cells (cd8/nkl), in the musculature of infected fish. These data support a role for cellular effectors such as antigen-presenting cells (monocyte/macrophage and dendritic cells) along with B and T cells in the acquired protective immune response of Atlantic salmon against K. thyrsites.
Keywords: Atlantic salmon; Kudoa thyrsites; antigen presentation; cytotoxicity; gene expression; immunohistochemistry; postmortem myoliquefaction.
Copyright © 2017 American Society for Microbiology.
Figures



References
-
- Moran JDW, Whitaker DJ, Kent ML. 1999. A review of the myxosporean genus Kudoa Meglitsch, 1947, and its impact on the international aquaculture industry and commercial fisheries. Aquaculture 172:163–196. doi: 10.1016/S0044-8486(98)00437-2. - DOI
-
- Dawson-Coates JA, Chase JC, Funk V, Booy MH, Haines LR, Falkenberg CL, Whitaker DJ, Olafson RW, Pearson TW. 2003. The relationship between flesh quality and numbers of Kudoa thyrsites plasmodia and spores in farmed Atlantic salmon, Salmo salar L. J Fish Dis 26:451–459. doi: 10.1046/j.1365-2761.2003.00477.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

