Pib2 and the EGO complex are both required for activation of TORC1
- PMID: 28993463
- PMCID: PMC5702048
- DOI: 10.1242/jcs.207910
Pib2 and the EGO complex are both required for activation of TORC1
Abstract
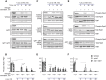
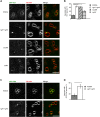
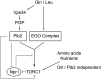
The TORC1 complex is a key regulator of cell growth and metabolism in Saccharomyces cerevisiae The vacuole-associated EGO complex couples activation of TORC1 to the availability of amino acids, specifically glutamine and leucine. The EGO complex is also essential for reactivation of TORC1 following rapamycin-induced growth arrest and for its distribution on the vacuolar membrane. Pib2, a FYVE-containing phosphatidylinositol 3-phosphate (PI3P)-binding protein, is a newly discovered and poorly characterized activator of TORC1. Here, we show that Pib2 is required for reactivation of TORC1 following rapamycin-induced growth arrest. Pib2 is required for EGO complex-mediated activation of TORC1 by glutamine and leucine as well as for redistribution of Tor1 on the vacuolar membrane. Therefore, Pib2 and the EGO complex cooperate to activate TORC1 and connect phosphoinositide 3-kinase (PI3K) signaling and TORC1 activity.
Keywords: EGO complex; Gtr1; Gtr2; Pib2; TORC1.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures



Similar articles
-
Gtr/Ego-independent TORC1 activation is achieved through a glutamine-sensitive interaction with Pib2 on the vacuolar membrane.PLoS Genet. 2018 Apr 26;14(4):e1007334. doi: 10.1371/journal.pgen.1007334. eCollection 2018 Apr. PLoS Genet. 2018. PMID: 29698392 Free PMC article.
-
The Vam6 GEF controls TORC1 by activating the EGO complex.Mol Cell. 2009 Sep 11;35(5):563-73. doi: 10.1016/j.molcel.2009.06.033. Mol Cell. 2009. PMID: 19748353
-
Dynamic relocation of the TORC1-Gtr1/2-Ego1/2/3 complex is regulated by Gtr1 and Gtr2.Mol Biol Cell. 2016 Jan 15;27(2):382-96. doi: 10.1091/mbc.E15-07-0470. Epub 2015 Nov 25. Mol Biol Cell. 2016. PMID: 26609069 Free PMC article.
-
Pib2 as an Emerging Master Regulator of Yeast TORC1.Biomolecules. 2021 Oct 9;11(10):1489. doi: 10.3390/biom11101489. Biomolecules. 2021. PMID: 34680122 Free PMC article. Review.
-
Conserved and Divergent Mechanisms That Control TORC1 in Yeasts and Mammals.Genes (Basel). 2021 Jan 12;12(1):88. doi: 10.3390/genes12010088. Genes (Basel). 2021. PMID: 33445779 Free PMC article. Review.
Cited by
-
Gtr/Ego-independent TORC1 activation is achieved through a glutamine-sensitive interaction with Pib2 on the vacuolar membrane.PLoS Genet. 2018 Apr 26;14(4):e1007334. doi: 10.1371/journal.pgen.1007334. eCollection 2018 Apr. PLoS Genet. 2018. PMID: 29698392 Free PMC article.
-
The molecular logic of Gtr1/2- and Pib2-dependent TORC1 regulation in budding yeast.Elife. 2025 Jul 7;13:RP94628. doi: 10.7554/eLife.94628. Elife. 2025. PMID: 40622848 Free PMC article.
-
Ivy1 is a negative regulator of Gtr-dependent TORC1 activation.J Cell Sci. 2018 Sep 7;131(17):jcs218305. doi: 10.1242/jcs.218305. J Cell Sci. 2018. PMID: 30097557 Free PMC article.
-
The role of Phafin proteins in cell signaling pathways and diseases.Open Life Sci. 2024 Jun 27;19(1):20220896. doi: 10.1515/biol-2022-0896. eCollection 2024. Open Life Sci. 2024. PMID: 38947768 Free PMC article. Review.
-
KAE1 Allelic Variants Affect TORC1 Activation and Fermentation Kinetics in Saccharomyces cerevisiae.Front Microbiol. 2019 Jul 31;10:1686. doi: 10.3389/fmicb.2019.01686. eCollection 2019. Front Microbiol. 2019. PMID: 31417508 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

