Mitochondrial Abnormality Facilitates Cyst Formation in Autosomal Dominant Polycystic Kidney Disease
- PMID: 28993480
- PMCID: PMC5705822
- DOI: 10.1128/MCB.00337-17
Mitochondrial Abnormality Facilitates Cyst Formation in Autosomal Dominant Polycystic Kidney Disease
Abstract
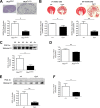
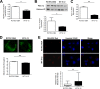
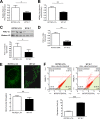
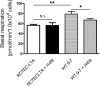
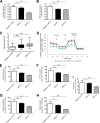
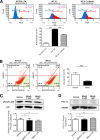
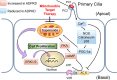
Autosomal dominant polycystic kidney disease (ADPKD) constitutes the most inherited kidney disease. Mutations in the PKD1 and PKD2 genes, encoding the polycystin 1 and polycystin 2 Ca2+ ion channels, respectively, result in tubular epithelial cell-derived renal cysts. Recent clinical studies demonstrate oxidative stress to be present early in ADPKD. Mitochondria comprise the primary reactive oxygen species source and also their main effector target; however, the pathophysiological role of mitochondria in ADPKD remains uncharacterized. To clarify this function, we examined the mitochondria of cyst-lining cells in ADPKD model mice (Ksp-Cre PKD1flox/flox) and rats (Han:SPRD Cy/+), demonstrating obvious tubular cell morphological abnormalities. Notably, the mitochondrial DNA copy number and peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α) expression were decreased in ADPKD model animal kidneys, with PGC-1α expression inversely correlated with oxidative stress levels. Consistent with these findings, human ADPKD cyst-derived cells with heterozygous and homozygous PKD1 mutation exhibited morphological and functional abnormalities, including increased mitochondrial superoxide. Furthermore, PGC-1α expression was suppressed by decreased intracellular Ca2+ levels via calcineurin, p38 mitogen-activated protein kinase (MAPK), and nitric oxide synthase deactivation. Moreover, the mitochondrion-specific antioxidant MitoQuinone (MitoQ) reduced intracellular superoxide and inhibited cyst epithelial cell proliferation through extracellular signal-related kinase/MAPK inactivation. Collectively, these results indicate that mitochondrial abnormalities facilitate cyst formation in ADPKD.
Keywords: mitochondrial metabolism; polycystic kidney disease.
Copyright © 2017 Ishimoto et al.
Figures








Similar articles
-
Cyst formation in kidney via B-Raf signaling in the PKD2 transgenic mice.J Biol Chem. 2009 Mar 13;284(11):7214-22. doi: 10.1074/jbc.M805890200. Epub 2008 Dec 20. J Biol Chem. 2009. PMID: 19098310 Free PMC article.
-
Adenylyl cyclase 5 links changes in calcium homeostasis to cAMP-dependent cyst growth in polycystic liver disease.J Hepatol. 2017 Mar;66(3):571-580. doi: 10.1016/j.jhep.2016.10.032. Epub 2016 Nov 5. J Hepatol. 2017. PMID: 27826057 Free PMC article.
-
Extracellular vesicles and exosomes generated from cystic renal epithelial cells promote cyst growth in autosomal dominant polycystic kidney disease.Nat Commun. 2021 Jul 27;12(1):4548. doi: 10.1038/s41467-021-24799-x. Nat Commun. 2021. PMID: 34315885 Free PMC article.
-
Possible role of the mitochondrial genome in the pathogenesis of autosomal dominant polycystic kidney disease.Nephrology (Carlton). 2021 Nov;26(11):920-930. doi: 10.1111/nep.13957. Epub 2021 Aug 9. Nephrology (Carlton). 2021. PMID: 34331378 Review.
-
Cell Proliferation and Apoptosis in ADPKD.Adv Exp Med Biol. 2016;933:25-34. doi: 10.1007/978-981-10-2041-4_3. Adv Exp Med Biol. 2016. PMID: 27730432 Review.
Cited by
-
Reactive oxygen species, toxicity, oxidative stress, and antioxidants: chronic diseases and aging.Arch Toxicol. 2023 Oct;97(10):2499-2574. doi: 10.1007/s00204-023-03562-9. Epub 2023 Aug 19. Arch Toxicol. 2023. PMID: 37597078 Free PMC article. Review.
-
The targeted anti-oxidant MitoQ causes mitochondrial swelling and depolarization in kidney tissue.Physiol Rep. 2018 Apr;6(7):e13667. doi: 10.14814/phy2.13667. Physiol Rep. 2018. PMID: 29611340 Free PMC article.
-
Shared pathobiology identifies AMPK as a therapeutic target for obesity and autosomal dominant polycystic kidney disease.Front Mol Biosci. 2022 Aug 23;9:962933. doi: 10.3389/fmolb.2022.962933. eCollection 2022. Front Mol Biosci. 2022. PMID: 36106024 Free PMC article. Review.
-
Potential Add-On Benefits of Dietary Intervention in the Treatment of Autosomal Dominant Polycystic Kidney Disease.Nutrients. 2024 Aug 6;16(16):2582. doi: 10.3390/nu16162582. Nutrients. 2024. PMID: 39203719 Free PMC article. Review.
-
Ketosis Ameliorates Renal Cyst Growth in Polycystic Kidney Disease.Cell Metab. 2019 Dec 3;30(6):1007-1023.e5. doi: 10.1016/j.cmet.2019.09.012. Epub 2019 Oct 17. Cell Metab. 2019. PMID: 31631001 Free PMC article.
References
-
- Chapman AB, Devuyst O, Eckardt KU, Gansevoort RT, Harris T, Horie S, Kasiske BL, Odland D, Pei Y, Perrone RD, Pirson Y, Schrier RW, Torra R, Torres VE, Watnick T, Wheeler DC, Conference Participants. 2015. Autosomal-dominant polycystic kidney disease (ADPKD): executive summary from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 88:17–27. doi:10.1038/ki.2015.59. - DOI - PMC - PubMed
-
- Hateboer N, van Dijk MA, Bogdanova N, Coto E, Saggar-Malik AK, San Millan JL, Torra R, Breuning M, Ravine D. 1999. Comparison of phenotypes of polycystic kidney disease types 1 and 2. European PKD1-PKD2 Study Group. Lancet 353:103–107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
