Interneurons in the Honeybee Primary Auditory Center Responding to Waggle Dance-Like Vibration Pulses
- PMID: 28993484
- PMCID: PMC6596516
- DOI: 10.1523/JNEUROSCI.0044-17.2017
Interneurons in the Honeybee Primary Auditory Center Responding to Waggle Dance-Like Vibration Pulses
Abstract
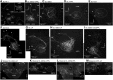
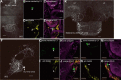
Female honeybees use the "waggle dance" to communicate the location of nectar sources to their hive mates. Distance information is encoded in the duration of the waggle phase (von Frisch, 1967). During the waggle phase, the dancer produces trains of vibration pulses, which are detected by the follower bees via Johnston's organ located on the antennae. To uncover the neural mechanisms underlying the encoding of distance information in the waggle dance follower, we investigated morphology, physiology, and immunohistochemistry of interneurons arborizing in the primary auditory center of the honeybee (Apis mellifera). We identified major interneuron types, named DL-Int-1, DL-Int-2, and bilateral DL-dSEG-LP, that responded with different spiking patterns to vibration pulses applied to the antennae. Experimental and computational analyses suggest that inhibitory connection plays a role in encoding and processing the duration of vibration pulse trains in the primary auditory center of the honeybee.SIGNIFICANCE STATEMENT The waggle dance represents a form of symbolic communication used by honeybees to convey the location of food sources via species-specific sound. The brain mechanisms used to decipher this symbolic information are unknown. We examined interneurons in the honeybee primary auditory center and identified different neuron types with specific properties. The results of our computational analyses suggest that inhibitory connection plays a role in encoding waggle dance signals. Our results are critical for understanding how the honeybee deciphers information from the sound produced by the waggle dance and provide new insights regarding how common neural mechanisms are used by different species to achieve communication.
Keywords: Johnston's organ; brain; dance language; honeybee; primary auditory center; vibration; waggle dance.
Copyright © 2017 the authors 0270-6474/17/3710624-12$15.00/0.
Figures






References
-
- Ai H, Itoh T (2012) The Auditory System of the Honeybee. In Honeybee neurobiology and behaviors, Ed 2 (Eisenhardt D, Galizia CG, Giurfa M, eds), pp 269–284. Berlin/Heidelberg, Germany: Springer.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
