Timing as a sexually selected trait: the right mate at the right moment
- PMID: 28993493
- PMCID: PMC5647276
- DOI: 10.1098/rstb.2016.0249
Timing as a sexually selected trait: the right mate at the right moment
Abstract
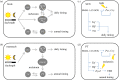
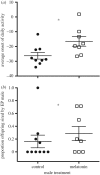
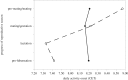
Sexual selection favours the expression of traits in one sex that attract members of the opposite sex for mating. The nature of sexually selected traits such as vocalization, colour and ornamentation, their fitness benefits as well as their costs have received ample attention in field and laboratory studies. However, sexually selected traits may not always be expressed: coloration and ornaments often follow a seasonal pattern and behaviours may be displayed only at specific times of the day. Despite the widely recognized differences in the daily and seasonal timing of traits and their consequences for reproductive success, the actions of sexual selection on the temporal organization of traits has received only scant attention. Drawing on selected examples from bird and mammal studies, here we summarize the current evidence for the daily and seasonal timing of traits. We highlight that molecular advances in chronobiology have opened exciting new opportunities for identifying the genetic targets that sexual selection may act on to shape the timing of trait expression. Furthermore, known genetic links between daily and seasonal timing mechanisms lead to the hypothesis that selection on one timescale may simultaneously also affect the other. We emphasize that studies on the timing of sexual displays of both males and females from wild populations will be invaluable for understanding the nature of sexual selection and its potential to act on differences within and between the sexes in timing. Molecular approaches will be important for pinpointing genetic components of biological rhythms that are targeted by sexual selection, and to clarify whether these represent core or peripheral components of endogenous clocks. Finally, we call for a renewed integration of the fields of evolution, behavioural ecology and chronobiology to tackle the exciting question of how sexual selection contributes to the evolution of biological clocks.This article is part of the themed issue 'Wild clocks: integrating chronobiology and ecology to understand timekeeping in free-living animals'.
Keywords: circadian rhythm; circannual rhythm; display behaviour; sexual selection; timing of reproduction.
© 2017 The Author(s).
Conflict of interest statement
We declare we have no competing interests.
Figures

Similar articles
-
Seasonal reproductive tactics: annual timing and the capital-to-income breeder continuum.Philos Trans R Soc Lond B Biol Sci. 2017 Nov 19;372(1734):20160250. doi: 10.1098/rstb.2016.0250. Philos Trans R Soc Lond B Biol Sci. 2017. PMID: 28993494 Free PMC article. Review.
-
Artificial selection reveals sex differences in the genetic basis of sexual attractiveness.Proc Natl Acad Sci U S A. 2018 May 22;115(21):5498-5503. doi: 10.1073/pnas.1720368115. Epub 2018 May 7. Proc Natl Acad Sci U S A. 2018. PMID: 29735676 Free PMC article.
-
Sexual selection, phenotypic plasticity and female reproductive output.Philos Trans R Soc Lond B Biol Sci. 2019 Mar 18;374(1768):20180184. doi: 10.1098/rstb.2018.0184. Philos Trans R Soc Lond B Biol Sci. 2019. PMID: 30966965 Free PMC article. Review.
-
Geographical and seasonal variation in the intensity of sexual selection in the barn swallow Hirundo rustica: a meta-analysis.Biol Rev Camb Philos Soc. 2017 Aug;92(3):1582-1600. doi: 10.1111/brv.12297. Epub 2016 Sep 12. Biol Rev Camb Philos Soc. 2017. PMID: 27615554
-
The role of ecology in speciation by sexual selection: a systematic empirical review.J Hered. 2014;105 Suppl 1:782-94. doi: 10.1093/jhered/esu037. J Hered. 2014. PMID: 25149254
Cited by
-
Long-term effects of noise pollution on the avian dawn chorus: a natural experiment facilitated by the closure of an international airport.Proc Biol Sci. 2022 Sep 14;289(1982):20220906. doi: 10.1098/rspb.2022.0906. Epub 2022 Sep 14. Proc Biol Sci. 2022. PMID: 36100015 Free PMC article.
-
Methods in field chronobiology.Philos Trans R Soc Lond B Biol Sci. 2017 Nov 19;372(1734):20160247. doi: 10.1098/rstb.2016.0247. Philos Trans R Soc Lond B Biol Sci. 2017. PMID: 28993491 Free PMC article. Review.
-
Sex-Related Variation in Circadian Rhythms in the Bumble Bee Bombus terrestris.J Biol Rhythms. 2024 Dec;39(6):594-606. doi: 10.1177/07487304241283863. Epub 2024 Oct 6. J Biol Rhythms. 2024. PMID: 39370745 Free PMC article.
-
Sex-specific expression of circadian rhythms enables allochronic speciation.Evol Lett. 2024 Oct 8;9(1):65-76. doi: 10.1093/evlett/qrae049. eCollection 2025 Feb. Evol Lett. 2024. PMID: 39906588 Free PMC article.
-
Seasonal patterns in behavior and glucocorticoid secretion of a specialist Holarctic tree squirrel (Sciurus aberti).J Comp Physiol B. 2022 Jul;192(3-4):541-559. doi: 10.1007/s00360-022-01429-6. Epub 2022 Feb 14. J Comp Physiol B. 2022. PMID: 35157127
References
-
- Arnold SJ. 1994. Bateman's principles and the measurement of sexual selection in plants and animals. Am. Nat. 144, S126–S149. (10.1086/285656) - DOI
-
- Darwin C. 1871. Sexual selection and the descent of man. London, UK: Murray.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

