Keeping time without a spine: what can the insect clock teach us about seasonal adaptation?
- PMID: 28993500
- PMCID: PMC5647283
- DOI: 10.1098/rstb.2016.0257
Keeping time without a spine: what can the insect clock teach us about seasonal adaptation?
Abstract
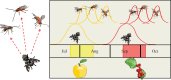
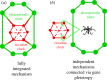
Seasonal change in daylength (photoperiod) is widely used by insects to regulate temporal patterns of development and behaviour, including the timing of diapause (dormancy) and migration. Flexibility of the photoperiodic response is critical for rapid shifts to new hosts, survival in the face of global climate change and to reproductive isolation. At the same time, the daily circadian clock is also essential for development, diapause and multiple behaviours, including correct flight orientation during long-distance migration. Although studied for decades, how these two critical biological timing mechanisms are integrated is poorly understood, in part because the core circadian clock genes are all transcription factors or regulators that are able to exert multiple effects throughout the genome. In this chapter, we discuss clocks in the wild from the perspective of diverse insect groups across eco-geographic contexts from the Antarctic to the tropical regions of Earth. Application of the expanding tool box of molecular techniques will lead us to distinguish universal from unique mechanisms underlying the evolution of circadian and photoperiodic timing, and their interaction across taxonomic and ecological contexts represented by insects.This article is part of the themed issue 'Wild clocks: integrating chronobiology and ecology to understand timekeeping in free-living animals'.
Keywords: climate change; clock genes; diapause; insect photoperiodism; migration; seasonal adaptations.
© 2017 The Author(s).
Conflict of interest statement
We declare we have no competing interests.
Figures

References
-
- Tauber MJ, Tauber CA, Masaki S. 1986. Seasonal adaptations in insects. Oxford, UK: Oxford University Press.
-
- Saunders DS. 2004. Insect clocks, 3rd edn Amsterdam, The Netherlands: Elsevier.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
