α-Synuclein impairs ferritinophagy in the retinal pigment epithelium: Implications for retinal iron dyshomeostasis in Parkinson's disease
- PMID: 28993630
- PMCID: PMC5634503
- DOI: 10.1038/s41598-017-12862-x
α-Synuclein impairs ferritinophagy in the retinal pigment epithelium: Implications for retinal iron dyshomeostasis in Parkinson's disease
Abstract
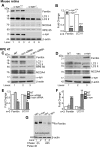
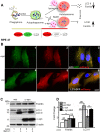
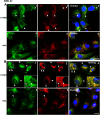
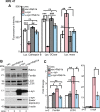
Retinal degeneration is prominent in Parkinson's disease (PD), a neuromotor disorder associated with aggregation of α-synuclein (α-syn) in the substantia-nigra (SN). Although α-syn is expressed in the neuroretina, absence of prominent aggregates suggests altered function as the likely cause of retinal pathology. We demonstrate that α-syn impairs ferritinophagy, resulting in the accumulation of iron-rich ferritin in the outer retina in-vivo and retinal-pigment-epithelial (RPE) cells in-vitro. Over-expression of Rab1a restores ferritinophagy, suggesting that α-syn impairs lysosomal function by disrupting the trafficking of lysosomal hydrolases. Surprisingly, upregulation of ferritin in RPE cells by exogenous iron in-vitro stimulated the release of ferritin and α-syn in exosomes, suggesting that iron overload due to impaired ferritinophagy or other cause(s) is likely to initiate prion-like spread of α-syn and ferritin, creating retinal iron dyshomeostasis and associated cytotoxicity. Since over-expression of α-syn is a known cause of PD, these results explain the likely cause of PD-associated retinal degeneration.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Alpha-synuclein modulates retinal iron homeostasis by facilitating the uptake of transferrin-bound iron: Implications for visual manifestations of Parkinson's disease.Free Radic Biol Med. 2016 Aug;97:292-306. doi: 10.1016/j.freeradbiomed.2016.06.025. Epub 2016 Jun 23. Free Radic Biol Med. 2016. PMID: 27343690 Free PMC article.
-
Prion protein facilitates retinal iron uptake and is cleaved at the β-site: Implications for retinal iron homeostasis in prion disorders.Sci Rep. 2017 Aug 29;7(1):9600. doi: 10.1038/s41598-017-08821-1. Sci Rep. 2017. PMID: 28851903 Free PMC article.
-
Neuropathological stages of neuronal, astrocytic and oligodendrocytic alpha-synuclein pathology in Parkinson's disease.Acta Neuropathol Commun. 2025 Feb 11;13(1):25. doi: 10.1186/s40478-025-01944-x. Acta Neuropathol Commun. 2025. PMID: 39934841 Free PMC article.
-
Biology, Pathology, and Targeted Therapy of Exosomal Cargoes in Parkinson's Disease: Advances and Challenges.Mol Neurobiol. 2025 Jul;62(7):8381-8399. doi: 10.1007/s12035-025-04788-7. Epub 2025 Feb 25. Mol Neurobiol. 2025. PMID: 39998798 Review.
-
Ferritinophagy and α-Synuclein: Pharmacological Targeting of Autophagy to Restore Iron Regulation in Parkinson's Disease.Int J Mol Sci. 2022 Feb 21;23(4):2378. doi: 10.3390/ijms23042378. Int J Mol Sci. 2022. PMID: 35216492 Free PMC article. Review.
Cited by
-
Iron-responsive-like elements and neurodegenerative ferroptosis.Learn Mem. 2020 Aug 17;27(9):395-413. doi: 10.1101/lm.052282.120. Print 2020 Sep. Learn Mem. 2020. PMID: 32817306 Free PMC article. Review.
-
The critical role of ferritinophagy in human disease.Front Pharmacol. 2022 Sep 8;13:933732. doi: 10.3389/fphar.2022.933732. eCollection 2022. Front Pharmacol. 2022. PMID: 36160450 Free PMC article. Review.
-
Untangling Tau and Iron: Exploring the Interaction Between Iron and Tau in Neurodegeneration.Front Mol Neurosci. 2018 Aug 17;11:276. doi: 10.3389/fnmol.2018.00276. eCollection 2018. Front Mol Neurosci. 2018. PMID: 30174587 Free PMC article. Review.
-
Iron mishandling in the brain and periphery in Parkinson's disease.NPJ Parkinsons Dis. 2025 Aug 18;11(1):246. doi: 10.1038/s41531-025-01089-7. NPJ Parkinsons Dis. 2025. PMID: 40825780 Free PMC article. Review.
-
Metal Chelation Therapy and Parkinson's Disease: A Critical Review on the Thermodynamics of Complex Formation between Relevant Metal Ions and Promising or Established Drugs.Biomolecules. 2019 Jul 9;9(7):269. doi: 10.3390/biom9070269. Biomolecules. 2019. PMID: 31324037 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

