Equiaxial Strain Modulates Adipose-derived Stem Cell Differentiation within 3D Biphasic Scaffolds towards Annulus Fibrosus
- PMID: 28993681
- PMCID: PMC5634474
- DOI: 10.1038/s41598-017-13240-3
Equiaxial Strain Modulates Adipose-derived Stem Cell Differentiation within 3D Biphasic Scaffolds towards Annulus Fibrosus
Abstract
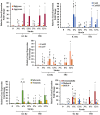
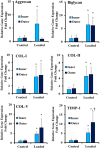
Recurrence of intervertebral disc (IVD) herniation is the most important factor leading to chronic low back pain and subsequent disability after discectomy. Efficacious annulus fibrosus (AF) repair strategy that delivers cells and biologics to IVD injury site is needed to limit the progression of disc degeneration and promote disc self-regeneration capacities after discectomy procedures. In this study, a biphasic mechanically-conditioned scaffold encapsulated with human adipose-derived stem cells (ASCs) is studied as a potential treatment strategy for AF defects. Equiaxial strains and frequencies were applied to ASCs-encapsulated scaffolds to identify the optimal loading modality to induce AF differentiation. Equiaxial loading resulted in 2-4 folds increase in secretion of extracellular matrix proteins and the reorganization of the matrix fibers and elongations of the cells along the load direction. Further, the equiaxial load induced region-specific differentiation of ASCs within the inner and outer regions of the biphasic scaffolds. Gene expression of AF markers was upregulated with 5-30 folds within the equiaxially loaded biphasic scaffolds compared to unstrained samples. The results suggest that there is a specific value of equiaxial strain favorable to differentiate ASCs towards AF lineage and that ASCs-embedded biphasic scaffold can potentially be utilized to repair the AF defects.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures







Similar articles
-
Differentiation of adipose stem cells seeded towards annulus fibrosus cells on a designed poly(trimethylene carbonate) scaffold prepared by stereolithography.J Tissue Eng Regen Med. 2017 Oct;11(10):2752-2762. doi: 10.1002/term.2170. Epub 2016 Jul 4. J Tissue Eng Regen Med. 2017. PMID: 27375236
-
Substrate stiffness- and topography-dependent differentiation of annulus fibrosus-derived stem cells is regulated by Yes-associated protein.Acta Biomater. 2019 Jul 1;92:254-264. doi: 10.1016/j.actbio.2019.05.013. Epub 2019 May 9. Acta Biomater. 2019. PMID: 31078765
-
Decellularized Annulus Fibrosus Matrix/Chitosan Hybrid Hydrogels with Basic Fibroblast Growth Factor for Annulus Fibrosus Tissue Engineering.Tissue Eng Part A. 2019 Dec;25(23-24):1605-1613. doi: 10.1089/ten.TEA.2018.0297. Epub 2019 Nov 21. Tissue Eng Part A. 2019. PMID: 30929614 Free PMC article.
-
Advanced Strategies for the Regeneration of Lumbar Disc Annulus Fibrosus.Int J Mol Sci. 2020 Jul 10;21(14):4889. doi: 10.3390/ijms21144889. Int J Mol Sci. 2020. PMID: 32664453 Free PMC article. Review.
-
Biomechanics in Annulus Fibrosus Degeneration and Regeneration.Adv Exp Med Biol. 2018;1078:409-420. doi: 10.1007/978-981-13-0950-2_21. Adv Exp Med Biol. 2018. PMID: 30357635 Review.
Cited by
-
Advancements in Degenerative Disc Disease Treatment: A Regenerative Medicine Approach.Stem Cell Rev Rep. 2025 Jun;21(5):1252-1282. doi: 10.1007/s12015-025-10882-z. Epub 2025 Apr 15. Stem Cell Rev Rep. 2025. PMID: 40232618 Review.
-
Extracellular Mechanical Stimuli Alters the Metastatic Progression of Prostate Cancer Cells within 3D Tissue Matrix.Bioengineering (Basel). 2023 Oct 31;10(11):1271. doi: 10.3390/bioengineering10111271. Bioengineering (Basel). 2023. PMID: 38002395 Free PMC article.
-
Application of stem cells combined with biomaterial in the treatment of intervertebral disc degeneration.Front Bioeng Biotechnol. 2022 Nov 25;10:1077028. doi: 10.3389/fbioe.2022.1077028. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36507272 Free PMC article. Review.
-
Synergetic role of TRPV4 inhibitor and mechanical loading on reducing inflammation.Front Immunol. 2025 Jan 7;15:1456042. doi: 10.3389/fimmu.2024.1456042. eCollection 2024. Front Immunol. 2025. PMID: 39850885 Free PMC article.
-
Putting the Pieces in Place: Mobilizing Cellular Players to Improve Annulus Fibrosus Repair.Tissue Eng Part B Rev. 2021 Aug;27(4):295-312. doi: 10.1089/ten.TEB.2020.0196. Epub 2020 Oct 19. Tissue Eng Part B Rev. 2021. PMID: 32907498 Free PMC article. Review.
References
-
- Wismer N, et al. Biodegradable electrospun scaffolds for annulus fibrosus tissue engineering: effect of scaffold structure and composition on annulus fibrosus cells in vitro. Tissue Eng Pt A. 2014;20:672–682. - PubMed
-
- Whatley BR, Wen XJ. Intervertebral disc (IVD): Structure, degeneration, repair and regeneration. Mat Sci Eng C-Mater. 2012;32:61–77. doi: 10.1016/j.msec.2011.10.011. - DOI
-
- Blanquer, S. B., Sharifi, S. & Grijpma, D. W. Development of poly (trimethylene carbonate) network implants for annulus fibrosus tissue engineering. J Appl Biomater Func10 (2012). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

