Dissecting the mycobacterial cell envelope and defining the composition of the native mycomembrane
- PMID: 28993692
- PMCID: PMC5634507
- DOI: 10.1038/s41598-017-12718-4
Dissecting the mycobacterial cell envelope and defining the composition of the native mycomembrane
Abstract
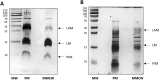
The mycobacterial envelope is unique, containing the so-called mycomembrane (MM) composed of very-long chain fatty acids, mycolic acids (MA). Presently, the molecular composition of the MM remains unproven, due to the diversity of methods used for determining its composition. The plasma membranes (PM) and the native MM-containing cell walls (MMCW) of two rapid-growing mycobacterial species, Mycobacterium aurum and M. smegmatis, were isolated from their cell lysates by differential ultracentrifugation. Transmission electron microscopy and biochemical analyses demonstrated that the two membranes were virtually pure. Bottom-up quantitative proteomics study indicated a different distribution of more than 2,100 proteins between the PM and MMCW. Among these, the mannosyltransferase PimB, galactofuranosyltransferase GlfT2, Cytochrome p450 and ABC transporter YjfF, were most abundant in the PM, which also contain lipoglycans, phospholipids, including phosphatidylinositol mannosides, and only a tiny amount of other glycolipids. Antigen85 complex proteins, porins and the putative transporters MCE protein family were mostly found in MMCW fraction that contains MA esterifying arabinogalactan, constituting the inner leaflet of MM. Glycolipids, phospholipids and lipoglycans, together with proteins, presumably composed the outer leaflet of the MM, a lipid composition that differs from that deduced from the widely used extraction method of mycobacterial cells with dioctylsulfosuccinate sodium.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- Wayne LG, Kubica GP. The Mycobacteria. The Bergey’s manual of systematic bacteriology. 1986;2:1435–1457.
-
- Brennan PJ, Goren MB. Structural studies on the type-specific antigens and lipids of the mycobacterium avium. Mycobacterium intracellulare. Mycobacterium scrofulaceum serocomplex. Mycobacterium intracellulare serotype 9. J Biol Chem. 1979;254:4205–4211. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

