Pathogenic Mechanisms of Bicuspid Aortic Valve Aortopathy
- PMID: 28993736
- PMCID: PMC5622294
- DOI: 10.3389/fphys.2017.00687
Pathogenic Mechanisms of Bicuspid Aortic Valve Aortopathy
Abstract
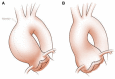
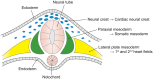
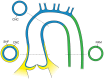
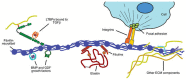
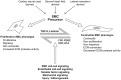
Bicuspid aortic valve (BAV) is the most common congenital valvular defect and is associated with ascending aortic dilation (AAD) in a quarter of patients. AAD has been ascribed both to the hemodynamic consequences of normally functioning and abnormal BAV morphology, and to the effect of rare and common genetic variation upon function of the ascending aortic media. AAD manifests in two overall and sometimes overlapping phenotypes: that of aortic root aneurysm, similar to the AAD of Marfan syndrome; and that of tubular AAD, similar to the AAD seen with tricuspid aortic valves (TAVs). These aortic phenotypes appear to be independent of BAV phenotype, have different embryologic origins and have unique etiologic factors, notably, regarding the role of hemodynamic changes inherent to the BAV phenotype. Further, in contrast to Marfan syndrome, the AAD seen with BAV is infrequently present as a strongly inherited syndromic phenotype; rather, it appears to be a less-penetrant, milder phenotype. Both reduced levels of normally functioning transcriptional proteins and structurally abnormal proteins have been observed in aneurysmal aortic media. We provide evidence that aortic root AAD has a stronger genetic etiology, sometimes related to identified common non-coding fibrillin-1 (FBN1) variants and other aortic wall protein variants in patients with BAV. In patients with BAV having tubular AAD, we propose a stronger hemodynamic influence, but with pathology still based on a functional deficit of the aortic media, of genetic or epigenetic etiology. Although it is an attractive hypothesis to ascribe common mechanisms to BAV and AAD, thus far the genetic etiologies of AAD have not been associated to the genetic etiologies of BAV, notably, not including BAV variants in NOTCH1 and GATA4.
Keywords: GATA4; bicuspid aortic valve; fibrillin; genetics; thoracic aortic aneurysm; transforming growth factor-β.
Figures





References
-
- 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines For the Diagnosis and Management of Patients with Thoracic Aortic Disease Representative Members. Hiratzka L. F., Creager M. A., Isselbacher E. M., Svensson L. G., 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease Representative Members. Nishimura R. A., et al. (2016). Surgery for aortic dilatation in patients with bicuspid aortic valves: a statement of clarification from the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 133, 680–686. 10.1161/CIR.0000000000000331 - DOI - PubMed
-
- Albinsson S., Della Corte A., Alajbegovic A., Krawczyk K. K., Bancone C., Galderisi U., et al. (2017). Patients with bicuspid and tricuspid aortic valve exhibit distinct regional microrna signatures in mildly dilated ascending aorta. Heart Vessels 32, 750–767. 10.1007/s00380-016-0942-7 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

