Trench Foot or Non-Freezing Cold Injury As a Painful Vaso-Neuropathy: Clinical and Skin Biopsy Assessments
- PMID: 28993756
- PMCID: PMC5626869
- DOI: 10.3389/fneur.2017.00514
Trench Foot or Non-Freezing Cold Injury As a Painful Vaso-Neuropathy: Clinical and Skin Biopsy Assessments
Abstract
Background: Trench foot, or non-freezing cold injury (NFCI), results from cold exposure of sufficient severity and duration above freezing point, with consequent sensory and vascular abnormalities which may persist for years. Based on observations of Trench foot in World War II, the condition was described as a vaso-neuropathy. While some reports have documented nerve damage after extreme cold exposure, sensory nerve fibres and vasculature have not been assessed with recent techniques in NFCI.
Objective: To assess patients with chronic sensory symptoms following cold exposure, in order to diagnose any underlying small fibre neuropathy, and provide insight into mechanisms of the persistent pain and cold hypersensitivity.
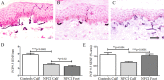
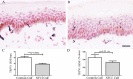
Methods: Thirty soldiers with cold exposure and persistent sensory symptoms (>4 months) were assessed with quantitative sensory testing, nerve conduction studies, and skin biopsies. Immunohistochemistry was used to assess intraepidermal (IENF) and subepidermal (SENF) nerve fibres with a range of markers, including the pan-neuronal marker protein gene product 9.5 (PGP 9.5), regenerating fibres with growth-associated protein 43 (GAP43), and nociceptor fibres with transient receptor potential cation channel subfamily V member 1 (TRPV1), sensory neuron-specific receptor (SNSR), and calcitonin gene-related peptide (CGRP). von Willebrand factor (vWF), endothelial nitric oxide synthase (eNOS), and vascular endothelial growth factor (VEGF) were used for assessing blood vessels, and transient receptor potential cation channel, subfamily A member 1 (TRPA1) and P2X purinoceptor 7 (P2X7) for keratinocytes, which regulate nociceptors via release of nerve growth factor.
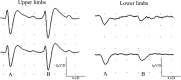
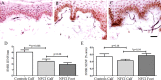
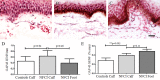
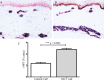
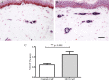
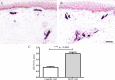
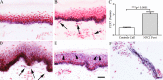
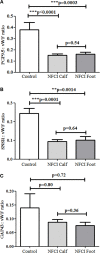
Results: Clinical examination showed pinprick sensation was abnormal in the feet of 20 patients (67%), and between 67 and 83% had abnormalities of thermal thresholds to the different modalities. 7 patients (23%) showed reduced sensory action potential amplitude of plantar nerves. 27 patients (90%) had decreased calf skin PGP 9.5 IENF (p < 0.0001), the remaining 3 patients had decreased nerve markers in subepidermis or foot skin. There were marked increases of all vascular markers (for vWF in calf skin, p < 0.0001), and increased sensory or regenerating SENF (for calf skin, GAP43, p = 0.002). TRPA1 (p = 0.0012) and P2X7 (p < 0.0001) were increased in basal keratinocytes.
Conclusion: A range of skin biopsy markers and plantar nerve conduction studies are useful objective assessments for the diagnosis of peripheral neuropathy in NFCI. Our results suggest that an increase in blood vessels following tissue ischaemia/hypoxia could be associated with disproportionate and abnormal nerve fibres (irritable nociceptors), and may lead to NFCI as a "painful vaso-neuropathy."
Keywords: nerve conduction study; neuropathy; non-freezing cold injury; pain; skin biopsy; trench foot.
Figures
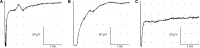



References
-
- Lorrain Smith J, Ritchie J, Dawson J. Clinical and experimental observations on the pathology of trench frostbite. J Pathol Bacteriol (1915) 20(1):159–90.10.1002/path.1700200113 - DOI
-
- Ungley CC, Blackwood W. Peripheral vasoneuropathy after chilling. “Immersion foot and immersion hand”. Lancet (1942) 240(6216):447–51.10.1016/S0140-6736(00)58135-5 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

