Monitoring the Secretory Behavior of the Rat Adrenal Medulla by High-Performance Liquid Chromatography-Based Catecholamine Assay from Slice Supernatants
- PMID: 28993760
- PMCID: PMC5622411
- DOI: 10.3389/fendo.2017.00248
Monitoring the Secretory Behavior of the Rat Adrenal Medulla by High-Performance Liquid Chromatography-Based Catecholamine Assay from Slice Supernatants
Abstract
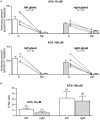
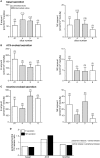
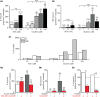
Catecholamine (CA) secretion from the adrenal medullary tissue is a key step of the adaptive response triggered by an organism to cope with stress. Whereas molecular and cellular secretory processes have been extensively studied at the single chromaffin cell level, data available for the whole gland level are much scarcer. We tackled this issue in rat by developing an easy to implement experimental strategy combining the adrenal acute slice supernatant collection with a high-performance liquid chromatography-based epinephrine and norepinephrine (NE) assay. This technique affords a convenient method for measuring basal and stimulated CA release from single acute slices, allowing thus to individually address the secretory function of the left and right glands. Our data point that the two glands are equally competent to secrete epinephrine and NE, exhibiting an equivalent epinephrine:NE ratio, both at rest and in response to a cholinergic stimulation. Nicotine is, however, more efficient than acetylcholine to evoke NE release. A pharmacological challenge with hexamethonium, an α3-containing nicotinic acetylcholine receptor antagonist, disclosed that epinephrine- and NE-secreting chromaffin cells distinctly expressed α3 nicotinic receptors, with a dominant contribution in NE cells. As such, beyond the novelty of CA assays from acute slice supernatants, our study contributes at refining the secretory behavior of the rat adrenal medullary tissue, and opens new perspectives for monitoring the release of other hormones and transmitters, especially those involved in the stress response.
Keywords: acetylcholine; acute adrenal slice; catecholamine release; fluorescence derivatization; hexamethonium; high-performance liquid chromatography; medullary tissue; stimulus-secretion coupling.
Figures
Similar articles
-
Arecoline inhibits catecholamine release from perfused rat adrenal gland.Acta Pharmacol Sin. 2006 Jan;27(1):71-9. doi: 10.1111/j.1745-7254.2006.00233.x. Acta Pharmacol Sin. 2006. PMID: 16364213
-
Regulation of catecholamine release in human adrenal chromaffin cells by β-adrenoceptors.Neurochem Int. 2012 Mar;60(4):387-93. doi: 10.1016/j.neuint.2011.12.018. Epub 2012 Jan 12. Neurochem Int. 2012. PMID: 22261351
-
Intrinsic gamma aminobutyric acid receptors modulate the release of catecholamine from canine adrenal gland in situ.J Pharmacol Exp Ther. 1986 Nov;239(2):584-90. J Pharmacol Exp Ther. 1986. PMID: 2877086
-
Influence of lobeline on catecholamine release from the isolated perfused rat adrenal gland.Auton Neurosci. 2004 Jan 30;110(1):27-35. doi: 10.1016/j.autneu.2003.10.001. Auton Neurosci. 2004. PMID: 14766322
-
In vivo demonstration of a paracrine, inhibitory action of Met-enkephalin on adrenomedullary catecholamine release in the rat.Endocrinology. 1989 Aug;125(2):624-9. doi: 10.1210/endo-125-2-624. Endocrinology. 1989. PMID: 2752969
Cited by
-
Effects of humeral intraosseous epinephrine in a pediatric hypovolemic cardiac arrest porcine model.Trauma Surg Acute Care Open. 2020 Feb 18;5(1):e000372. doi: 10.1136/tsaco-2019-000372. eCollection 2020. Trauma Surg Acute Care Open. 2020. PMID: 32154374 Free PMC article.
-
Nicotine and vascular dysfunction.Acta Physiol (Oxf). 2021 Apr;231(4):e13631. doi: 10.1111/apha.13631. Epub 2021 Feb 24. Acta Physiol (Oxf). 2021. PMID: 33595878 Free PMC article. Review.
-
Partial Agonist Activity of Neonicotinoids on Rat Nicotinic Receptors: Consequences over Epinephrine Secretion and In Vivo Blood Pressure.Int J Mol Sci. 2021 May 12;22(10):5106. doi: 10.3390/ijms22105106. Int J Mol Sci. 2021. PMID: 34065933 Free PMC article.
-
Adaptive remodeling of rat adrenomedullary stimulus-secretion coupling in a chronic hypertensive environment.Cell Mol Life Sci. 2024 Dec 27;82(1):31. doi: 10.1007/s00018-024-05524-5. Cell Mol Life Sci. 2024. PMID: 39725761 Free PMC article.
-
Innervated adrenomedullary microphysiological system to model nicotine and opioid exposure.Organs Chip. 2021 Nov;3:100009. doi: 10.1016/j.ooc.2021.100009. Epub 2021 Oct 22. Organs Chip. 2021. PMID: 38650595 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

