Rescuing Self: Transient Isolation and Autologous Transplantation of Bone Marrow Mitigates Radiation-Induced Hematopoietic Syndrome and Mortality in Mice
- PMID: 28993772
- PMCID: PMC5622201
- DOI: 10.3389/fimmu.2017.01180
Rescuing Self: Transient Isolation and Autologous Transplantation of Bone Marrow Mitigates Radiation-Induced Hematopoietic Syndrome and Mortality in Mice
Abstract
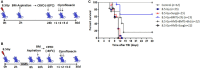
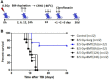
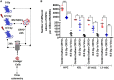
The inflamed bone marrow niche shortly after total body irradiation (TBI) is known to contribute to loss of hematopoietic stem cells in terms of their number and function. In this study, autologous bone marrow transfer (AL-BMT) was evaluated as a strategy for mitigating hematopoietic form of the acute radiation syndrome by timing the collection phase (2 h after irradiation) and reinfusion (24 h after irradiation) using mice as a model system. Collection of bone marrow (BM) cells (0.5 × 106 total marrow cells) 2 h after lethal TBI rescued different subclasses of hematopoietic stem and progenitor cells (HSPCs) from the detrimental inflammatory and damaging milieu in vivo. Cryopreservation of collected graft and its reinfusion 24 h after TBI significantly rescued mice from lethal effects of irradiation (65% survival against 0% in TBI group on day 30th) and hematopoietic depression. Transient hypometabolic state (HMS) induced 2 h after TBI effectively preserved the functional status of HSPCs and improved hematopoietic recovery even when BM was collected 8 h after TBI. Homing studies suggested that AL-BMT yielded similar percentages for different subsets of HSPCs when compared to syngeneic bone marrow transfer. The results suggest that the timing of collection, and reinfusion of graft is crucial for the success of AL-BMT.
Keywords: autologous bone marrow transfer; hematopoietic stem cells; hematopoietic syndrome; homing; ionizing radiation; mitigation.
Figures



References
-
- Champlin R. The role of bone marrow transplantation for nuclear accidents: implications of the Chernobyl disaster. Semin Hematol (1987) 24:1–4. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

