Spontaneous Secretion of the Citrullination Enzyme PAD2 and Cell Surface Exposure of PAD4 by Neutrophils
- PMID: 28993780
- PMCID: PMC5622307
- DOI: 10.3389/fimmu.2017.01200
Spontaneous Secretion of the Citrullination Enzyme PAD2 and Cell Surface Exposure of PAD4 by Neutrophils
Abstract
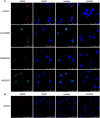
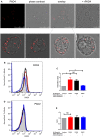
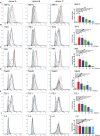
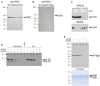
Autoantibodies directed against citrullinated epitopes of proteins are highly diagnostic of rheumatoid arthritis (RA), and elevated levels of protein citrullination can be found in the joints of patients with RA. Calcium-dependent peptidyl-arginine deiminases (PAD) are the enzymes responsible for citrullination. PAD2 and PAD4 are enriched in neutrophils and likely drive citrullination under inflammatory conditions. PADs may be released during NETosis or cell death, but the mechanisms responsible for PAD activity under physiological conditions have not been fully elucidated. To understand how PADs citrullinate extracellular proteins, we investigated the cellular localization and activity of PAD2 and PAD4, and we report that viable neutrophils from healthy donors have active PAD4 exposed on their surface and spontaneously secrete PAD2. Neutrophil activation by some stimulatory agents increased the levels of immunoreactive PAD4 on the cell surface, and some stimuli reduced PAD2 secretion. Our data indicate that live neutrophils have the inherent capacity to express active extracellular PADs. These novel pathways are distinguished from intracellular PAD activation during NETosis and calcium influx-mediated hypercitrullination. Our study implies that extracellular PADs may have a physiological role under non-pathogenic conditions as well as a pathological role in RA.
Keywords: PAD2; PAD4; citrullination; neutrophil; rheumatoid arthritis.
Figures


Similar articles
-
Peptidylarginine deiminase 2 is required for tumor necrosis factor alpha-induced citrullination and arthritis, but not neutrophil extracellular trap formation.J Autoimmun. 2017 Jun;80:39-47. doi: 10.1016/j.jaut.2017.01.006. Epub 2017 Feb 7. J Autoimmun. 2017. PMID: 28188029 Free PMC article.
-
The vacuolar anti-Pseudomonal activity of neutrophil primary granule peptidyl-arginine deiminase enzymes.Front Immunol. 2024 Oct 18;15:1452393. doi: 10.3389/fimmu.2024.1452393. eCollection 2024. Front Immunol. 2024. PMID: 39493757 Free PMC article.
-
Protein Arginine Deiminases (PADs): Biochemistry and Chemical Biology of Protein Citrullination.Acc Chem Res. 2019 Mar 19;52(3):818-832. doi: 10.1021/acs.accounts.9b00024. Epub 2019 Mar 7. Acc Chem Res. 2019. PMID: 30844238 Free PMC article.
-
PAD enzymes in rheumatoid arthritis: pathogenic effectors and autoimmune targets.Nat Rev Rheumatol. 2020 Jun;16(6):301-315. doi: 10.1038/s41584-020-0409-1. Epub 2020 Apr 27. Nat Rev Rheumatol. 2020. PMID: 32341463 Review.
-
PADs and NETs in digestive system: From physiology to pathology.Front Immunol. 2023 Jan 24;14:1077041. doi: 10.3389/fimmu.2023.1077041. eCollection 2023. Front Immunol. 2023. PMID: 36761761 Free PMC article. Review.
Cited by
-
No NETs no TIME: Crosstalk between neutrophil extracellular traps and the tumor immune microenvironment.Front Immunol. 2022 Dec 23;13:1075260. doi: 10.3389/fimmu.2022.1075260. eCollection 2022. Front Immunol. 2022. PMID: 36618417 Free PMC article. Review.
-
Oral mucosal breaks trigger anti-citrullinated bacterial and human protein antibody responses in rheumatoid arthritis.Sci Transl Med. 2023 Feb 22;15(684):eabq8476. doi: 10.1126/scitranslmed.abq8476. Epub 2023 Feb 22. Sci Transl Med. 2023. PMID: 36812347 Free PMC article.
-
Thioredoxin Modulates Protein Arginine Deiminase 4 (PAD4)-Catalyzed Citrullination.Front Immunol. 2019 Feb 19;10:244. doi: 10.3389/fimmu.2019.00244. eCollection 2019. Front Immunol. 2019. PMID: 30853960 Free PMC article.
-
A Hairy Cituation - PADIs in Regeneration and Alopecia.Front Cell Dev Biol. 2021 Dec 13;9:789676. doi: 10.3389/fcell.2021.789676. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34966743 Free PMC article. Review.
-
Citrullinated fibrinogen-SAAs complex causes vascular metastagenesis.Nat Commun. 2023 Aug 24;14(1):4960. doi: 10.1038/s41467-023-40371-1. Nat Commun. 2023. PMID: 37620307 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

