Delta Opioid Receptor Expression and Function in Primary Afferent Somatosensory Neurons
- PMID: 28993838
- PMCID: PMC7348656
- DOI: 10.1007/164_2017_58
Delta Opioid Receptor Expression and Function in Primary Afferent Somatosensory Neurons
Abstract
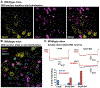
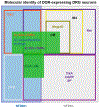
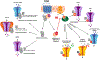
The functional diversity of primary afferent neurons of the dorsal root ganglia (DRG) generates a variety of qualitatively and quantitatively distinct somatosensory experiences, from shooting pain to pleasant touch. In recent years, the identification of dozens of genetic markers specifically expressed by subpopulations of DRG neurons has dramatically improved our understanding of this diversity and provided the tools to manipulate their activity and uncover their molecular identity and function. Opioid receptors have long been known to be expressed by discrete populations of DRG neurons, in which they regulate cell excitability and neurotransmitter release. We review recent insights into the identity of the DRG neurons that express the delta opioid receptor (DOR) and the ion channel mechanisms that DOR engages in these cells to regulate sensory input. We highlight recent findings derived from DORGFP reporter mice and from in situ hybridization and RNA sequencing studies in wild-type mice that revealed DOR presence in cutaneous mechanosensory afferents eliciting touch and implicated in tactile allodynia. Mechanistically, we describe how DOR modulates opening of voltage-gated calcium channels (VGCCs) to control glutamatergic neurotransmission between somatosensory neurons and postsynaptic neurons in the spinal cord dorsal horn. We additionally discuss other potential signaling mechanisms, including those involving potassium channels, which DOR may engage to fine tune somatosensation. We conclude by discussing how this knowledge may explain the analgesic properties of DOR agonists against mechanical pain and uncovers an unanticipated specialized function for DOR in cutaneous mechanosensation.
Keywords: Delta opioid receptor; Excitability; Ion channels; Mechanosensation; Neuroanatomy; Neurotransmitter release; Pain; Primary afferent dorsal root ganglion neurons; Touch.
Figures

References
-
- Afify EA, Khedr MM, Omar AG, Nasser SA (2013) The involvement of K(ATP) channels in morphine-induced antinociception and hepatic oxidative stress in acute and inflammatory pain in rats. Fundam Clin Pharmacol 27:623–631 - PubMed
-
- Aguilar-Bryan L et al. (1998) Toward understanding the assembly and structure of KATP channels. Physiol Rev 78:227–245 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

