Drug-eluting bead transarterial chemoembolization in the treatment for unresectable soft tissue sarcoma refractory to systemic chemotherapy: a preliminary evaluation of efficacy and safety
- PMID: 28993945
- PMCID: PMC11813283
- DOI: 10.1007/s00432-017-2530-3
Drug-eluting bead transarterial chemoembolization in the treatment for unresectable soft tissue sarcoma refractory to systemic chemotherapy: a preliminary evaluation of efficacy and safety
Abstract
Purpose: To preliminarily evaluate the clinical efficacy and safety of drug-eluting bead transarterial chemoembolization (DEB-TACE) for unresectable soft tissue sarcoma refractory to systemic chemotherapy.
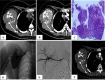
Methods: Ten patients with refractory sarcoma who underwent DEB-TACE therapy between January 2015 and January 2017 were identified. Clinical information and radiological data were retrospectively collected to analyze tumor response, overall survival (OS), progression-free survival and adverse events (AEs). Tumor response to DEB-TACE was assessed with modified Response Evaluation Criteria in Solid Tumors (mRECIST) guidelines applied to computed tomography or magnetic resonance imaging.
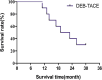
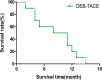
Results: All DEB-TACE procedures were successfully performed for ten patients with 15 tumor lesions. The median follow-up duration was 19 months and the median survival time was 21 months (range 11-30 months). The 1- and 2-year OS rate was 90 and 30%, respectively. According to the guidance of mRECIST, complete response, partial response, stable disease and progressive disease were noted in zero (0%), three (30%), four (40%) and three (30%) patients, respectively. The disease control rate and objective response rate was 70 and 30%, respectively. There were no serious AEs in patients after DEB-TACE.
Conclusions: Our data showed that DEB-TACE was effective and safe for patients with soft tissue sarcoma. Therefore, DEB-TACE can be considered as an alternative treatment option for unresectable soft tissue sarcoma refractory to conventionally systemic chemotherapy.
Keywords: Drug-eluting bead; Soft tissue sarcoma; Systemic chemotherapy; Transarterial chemoembolization.
Conflict of interest statement
We declare that we have no conflict of interest.
Figures
References
-
- Chawla SP, Papai Z, Mukhametshina G et al (2015) First-line aldoxorubicin vs doxorubicin in metastatic or locally advanced unresectable soft-tissue sarcoma: a phase 2b randomized clinical trial. JAMA Oncol 1:1272–1280 - PubMed
-
- Constantinidou A, van der Graaf WTA (2017) The fate of new fosfamides in phase III studies in advanced soft tissue sarcoma. Eur J Cancer 84:257–261 - PubMed
-
- Facciorusso A, Mariani L, Sposito C et al (2016) Drug-eluting beads versus conventional chemoembolization for the treatment of unresectable hepatocellular carcinoma. J Gastroenterol Hepatol 31:645–653 - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

