Methamphetamine Augments Concurrent Astrocyte Mitochondrial Stress, Oxidative Burden, and Antioxidant Capacity: Tipping the Balance in HIV-Associated Neurodegeneration
- PMID: 28993979
- PMCID: PMC6003420
- DOI: 10.1007/s12640-017-9812-z
Methamphetamine Augments Concurrent Astrocyte Mitochondrial Stress, Oxidative Burden, and Antioxidant Capacity: Tipping the Balance in HIV-Associated Neurodegeneration
Abstract
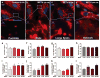
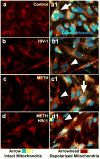
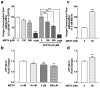
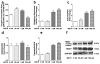
Methamphetamine (METH) use, with and without human immunodeficiency virus (HIV)-1 comorbidity, exacerbates neurocognitive decline. Oxidative stress is a probable neurotoxic mechanism during HIV-1 central nervous system infection and METH abuse, as viral proteins, antiretroviral therapy and METH have each been shown to induce mitochondrial dysfunction. However, the mechanisms regulating mitochondrial homeostasis and overall oxidative burden in astrocytes are not well understood in the context of HIV-1 infection and METH abuse. Here, we report METH-mediated dysregulation of astrocyte mitochondrial morphology and function during prolonged exposure to low levels of METH. Mitochondria became larger and more rod shaped with METH when assessed by machine learning, segmentation analyses. These changes may be mediated by elevated mitofusin expression coupled with inhibitory phosphorylation of dynamin-related protein-1, which regulate mitochondrial fusion and fission, respectively. While METH decreased oxygen consumption and ATP levels during acute exposure, chronic treatment of 1 to 2 weeks significantly enhanced both when tested in the absence of METH. Together, these changes significantly increased not only expression of antioxidant proteins, augmenting the astrocyte's oxidative capacity, but also oxidative damage. We propose that targeting astrocytes to reduce their overall oxidative burden and expand their antioxidant capacity could ultimately tip the balance from neurotoxicity towards neuroprotection.
Keywords: Astroglia; Dynamin-related protein; Extracellular flux; Machine learning; Mitochondria; Mitofusin; Neurotoxicity; Oxidative stress.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

