High frequency of brain metastases after adjuvant therapy for high-risk melanoma
- PMID: 28994212
- PMCID: PMC5673911
- DOI: 10.1002/cam4.1223
High frequency of brain metastases after adjuvant therapy for high-risk melanoma
Abstract
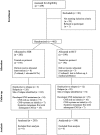
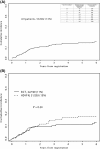
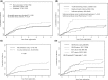
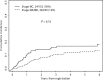
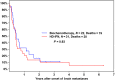
The incidence of CNS progression in patients with high-risk regional melanoma (stages IIIAN2a-IIIC) is not well characterized. Data from the S0008 trial provided an opportunity to examine the role of CNS progression in treatment failure and survival. All patients were surgically staged. Following wide excision and full regional lymphadenectomy, patients were randomized to receive adjuvant biochemotherapy (BCT) or high-dose interferon alfa-2B (HDI). CNS progression was retrospectively identified from data forms. Survival was measured from date of CNS progression. A total of 402 eligible patients were included in the analysis (BCT: 199, HDI: 203). Median follow-up (if alive) was over 7 years (range: 1 month to 11 years). The site of initial progression was identifiable in 80% of relapsing patients. CNS progression was a component of systemic melanoma relapse in 59/402 patients (15% overall). In 34/402 patients (9%) CNS progression represented the initial site of treatment failure. CNS progression was a component of initial progression in 27% of all patients whose melanoma relapsed (59/221). The risk of CNS progression was highest within 3 years of randomization. The difference in CNS progression rates between treatment arms was not significant (BCT = 25, HDI = 34, P = 0.24). Lymph node macrometastases strongly associated with CNS progression (P = 0.001), while ulceration and head and neck primaries were not significant predictors. This retrospective analysis of the S0008 trial identified a high brain metastasis rate (15%) in regionally advanced melanoma patients. Further studies are needed to establish whether screening plus earlier treatment would improve survival following CNS progression.
Keywords: Biochemotherapy; brain metastases; interferon; lymph node metastases; melanoma; ulceration.
© 2017 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Figures
References
-
- Sul, J. , and Posner J. B.. 2007. Brain metastases: epidemiology and pathophysiology. Cancer Treat. Res. 136:1–21. - PubMed
-
- Wen, P. Y , P. M., Black , and Loeffler J. S.. 2001. Metastatic Brain Cancer Pp.2655–2670 in DeVita V.T., Hellman S, Rosenberg S.A., eds. Cancer: Principles & Practice of Oncology. Philadelphia: Lippincott, Williams & Wilkins.
-
- Samlowski, W. E. , Watson G. A., Wang M., Rao G., Klimo P., Boucher K. Jr., et al. 2007. Multimodality treatment of melanoma brain metastases incorporating stereotactic radiosurgery (SRS). Cancer 109:1855–1862. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- N01 CA004919/CA/NCI NIH HHS/United States
- U10 CA022433/CA/NCI NIH HHS/United States
- U10 CA027057/CA/NCI NIH HHS/United States
- N01 CA035176/CA/NCI NIH HHS/United States
- U10 CA012644/CA/NCI NIH HHS/United States
- U10 CA037981/CA/NCI NIH HHS/United States
- U10 CA004919/CA/NCI NIH HHS/United States
- U10 CA046113/CA/NCI NIH HHS/United States
- U10 CA045560/CA/NCI NIH HHS/United States
- U10 CA076447/CA/NCI NIH HHS/United States
- N01 CA032102/CA/NCI NIH HHS/United States
- N01 CA013612/CA/NCI NIH HHS/United States
- U10 CA045808/CA/NCI NIH HHS/United States
- U10 CA021115/CA/NCI NIH HHS/United States
- U10 CA013612/CA/NCI NIH HHS/United States
- U10 CA031946/CA/NCI NIH HHS/United States
- U10 CA058416/CA/NCI NIH HHS/United States
- U10 CA014028/CA/NCI NIH HHS/United States
- U10 CA035281/CA/NCI NIH HHS/United States
- U10 CA080775/CA/NCI NIH HHS/United States
- U10 CA035261/CA/NCI NIH HHS/United States
- U10 CA035178/CA/NCI NIH HHS/United States
- U10 CA032102/CA/NCI NIH HHS/United States
- U10 CA046282/CA/NCI NIH HHS/United States
- U10 CA035262/CA/NCI NIH HHS/United States
- N01 CA035178/CA/NCI NIH HHS/United States
- N01 CA038926/CA/NCI NIH HHS/United States
- U10 CA067575/CA/NCI NIH HHS/United States
- N01 CA027057/CA/NCI NIH HHS/United States
- U10 CA058686/CA/NCI NIH HHS/United States
- U10 CA045377/CA/NCI NIH HHS/United States
- U10 CA020319/CA/NCI NIH HHS/United States
- U10 CA098543/CA/NCI NIH HHS/United States
- U10 CA038926/CA/NCI NIH HHS/United States
- U10 CA086780/CA/NCI NIH HHS/United States
- U10 CA007190/CA/NCI NIH HHS/United States
- U10 CA074811/CA/NCI NIH HHS/United States
- U10 CA011083/CA/NCI NIH HHS/United States
- U10 CA046368/CA/NCI NIH HHS/United States
- N01 CA067575/CA/NCI NIH HHS/United States
- U10 CA067663/CA/NCI NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
- U10 CA180819/CA/NCI NIH HHS/United States
- U10 CA035176/CA/NCI NIH HHS/United States
- U10 CA035090/CA/NCI NIH HHS/United States
- U10 CA058861/CA/NCI NIH HHS/United States
- N01 CA045560/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

