Selective Activation of C-H Bonds in a Cascade Process Combining Photochemistry and Biocatalysis
- PMID: 28994504
- PMCID: PMC5725739
- DOI: 10.1002/anie.201708668
Selective Activation of C-H Bonds in a Cascade Process Combining Photochemistry and Biocatalysis
Abstract
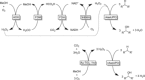
Selective oxyfunctionalizations of inert C-H bonds can be achieved under mild conditions by using peroxygenases. This approach, however, suffers from the poor robustness of these enzymes in the presence of hydrogen peroxide as the stoichiometric oxidant. Herein, we demonstrate that inorganic photocatalysts such as gold-titanium dioxide efficiently provide H2 O2 through the methanol-driven reductive activation of ambient oxygen in amounts that ensure that the enzyme remains highly active and stable. Using this approach, the stereoselective hydroxylation of ethylbenzene to (R)-1-phenylethanol was achieved with high enantioselectivity (>98 % ee) and excellent turnover numbers for the biocatalyst (>71 000).
Keywords: Biocatalysis; TiO2; oxyfunctionalization; peroxygenases; photocatalysis.
© 2017 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- None
-
- Wang Y., Lan D., Durrani R., Hollmann F., Curr. Opin. Chem. Biol. 2017, 37, 1–9; - PubMed
-
- Bormann S., Gomez Baraibar A., Ni Y., Holtmann D., Hollmann F., Catal. Sci. Technol. 2015, 5, 2038–2052.
-
- Hofrichter M., Ullrich R., Curr. Opin. Chem. Biol. 2014, 19, 116–125. - PubMed
-
- Ni Y., Fernández-Fueyo E., Baraibar A. G., Ullrich R., Hofrichter M., Yanase H., Alcalde M., van Berkel W. J. H., Hollmann F., Angew. Chem. Int. Ed. 2016, 55, 798–801; - PubMed
- Angew. Chem. 2016, 128, 809–812.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

