Characterization of Mucosal Disaccharidases from Human Intestine
- PMID: 28994704
- PMCID: PMC5691722
- DOI: 10.3390/nu9101106
Characterization of Mucosal Disaccharidases from Human Intestine
Abstract
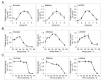
In this study, we used a brush border membrane (BBM) preparation from human small intestine to analyze the proportion and the activity of major intestinal disaccharidases, including sucrase-isomaltase (SI), maltase-glucoamylase (MGAM) and lactase-phlorizin hydrolase (LPH). SI, MGAM and LPH respectively constituted 8.2%, 2.7% and 1.4% of total BBM protein. The activity of SI and LPH decreased threefold after purification from the brush border membrane, which highlights the effect of membrane microdomains on the functional capacity of these enzymes. All of the disaccharidases showed optimal activity at pH 6, over 50% residual activity between pH 5 to pH 7, and increasing activity with rising temperatures up to 45 °C, along with a stable functional structure. Therefore the enzymes can withstand mild intraluminal pH alterations with adequate function, and are able to increase their activity with elevated core body temperature. Our data provide a functional measure for characterization of intestinal disaccharidases under different physiological and pathological conditions.
Keywords: enzyme activity; intestinal disaccharidases; pH profile; thermal activity profile.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Naim H.Y., Zimmer K.-P. Genetically determined disaccharidase deficiency. In: Kleinman R., Goulet O.-J., Mieli-Vergani G., Sanderson I., Sherman P., Shneider B., editors. Walker’s Pediatric Gastrointestinal Disease. Volume 2. PMPH-USA; Orlando, FL, USA: 2008. pp. 880–887.
-
- Naim H.Y., Sterchi E.E., Lentze M.J. Biosynthesis of the human sucrase-isomaltase complex-differential O-glycosylation of the sucrase subunit correlates with its position within the enzyme complex. J. Biol. Chem. 1988;263:7242–7253. - PubMed
-
- Naim H.Y., Sterchi E.E., Lentze M.J. Structure, biosynthesis, and glycosylation of human small intestinal maltase-glucoamylase. J. Biol. Chem. 1988;263:19709–19717. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

