Synthesis of 11C-Labelled Ureas by Palladium(II)-Mediated Oxidative Carbonylation
- PMID: 28994734
- PMCID: PMC6151465
- DOI: 10.3390/molecules22101688
Synthesis of 11C-Labelled Ureas by Palladium(II)-Mediated Oxidative Carbonylation
Abstract
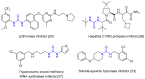
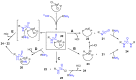
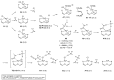
Positron emission tomography is an imaging technique with applications in clinical settings as well as in basic research for the study of biological processes. A PET tracer, a biologically active molecule where a positron-emitting radioisotope such as carbon-11 has been incorporated, is used for the studies. Development of robust methods for incorporation of the radioisotope is therefore of the utmost importance. The urea functional group is present in many biologically active compounds and is thus an attractive target for incorporation of carbon-11 in the form of [11C]carbon monoxide. Starting with amines and [11C]carbon monoxide, both symmetrical and unsymmetrical 11C-labelled ureas were synthesised via a palladium(II)-mediated oxidative carbonylation and obtained in decay-corrected radiochemical yields up to 65%. The added advantage of using [11C]carbon monoxide was shown by the molar activity obtained for an inhibitor of soluble epoxide hydrolase (247 GBq/μmol-319 GBq/μmol). DFT calculations were found to support a reaction mechanism proceeding through an 11C-labelled isocyanate intermediate.
Keywords: 11C-labelling; carbon monoxide; carbon-11; carbonylation; positron emission tomography; urea.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Palladium-mediated oxidative carbonylation reactions for the synthesis of (11) C-radiolabelled ureas.J Labelled Comp Radiopharm. 2014 Apr;57(4):202-8. doi: 10.1002/jlcr.3151. Epub 2013 Dec 11. J Labelled Comp Radiopharm. 2014. PMID: 24327390
-
[(11)C] Carbon monoxide in selenium-mediated synthesis of (11)C-carbamoyl compounds.J Org Chem. 2002 May 31;67(11):3687-92. doi: 10.1021/jo016307d. J Org Chem. 2002. PMID: 12027681
-
Palladium-catalyzed N-acylation of monosubstituted ureas using near-stoichiometric carbon monoxide.J Org Chem. 2012 Apr 20;77(8):3793-9. doi: 10.1021/jo3000767. Epub 2012 Apr 10. J Org Chem. 2012. PMID: 22458554
-
Transition metal mediated [(11) C]carbonylation reactions: recent advances and applications.J Labelled Comp Radiopharm. 2014 Apr;57(4):195-201. doi: 10.1002/jlcr.3150. Epub 2014 Jan 14. J Labelled Comp Radiopharm. 2014. PMID: 24425679 Review.
-
[(11) C]Carbon monoxide in labeling chemistry and positron emission tomography tracer development: scope and limitations.J Labelled Comp Radiopharm. 2015 Mar;58(3):86-98. doi: 10.1002/jlcr.3262. Epub 2015 Feb 16. J Labelled Comp Radiopharm. 2015. PMID: 25689679 Review.
Cited by
-
Improved synthesis of SV2A targeting radiotracer [11C]UCB-J.EJNMMI Radiopharm Chem. 2019 Nov 29;4(1):30. doi: 10.1186/s41181-019-0080-5. EJNMMI Radiopharm Chem. 2019. PMID: 31784919 Free PMC article.
-
Palladium-Mediated Synthesis of [Carbonyl-11C]acyl Amidines from Aryl Iodides and Aryl Bromides and Their One-Pot Cyclization to 11C-Labeled Oxadiazoles.J Org Chem. 2023 Apr 21;88(8):5118-5126. doi: 10.1021/acs.joc.2c02102. Epub 2022 Dec 13. J Org Chem. 2023. PMID: 36512765 Free PMC article.
-
Broad Scope and High-Yield Access to Unsymmetrical Acyclic [11 C]Ureas for Biomedical Imaging from [11 C]Carbonyl Difluoride.Chemistry. 2021 Jul 16;27(40):10369-10376. doi: 10.1002/chem.202100690. Epub 2021 Jun 2. Chemistry. 2021. PMID: 33890705 Free PMC article.
-
A [11 C] CO dispensing system for rapid screening of carbonylation reactions.J Labelled Comp Radiopharm. 2018 Dec;61(14):1110-1114. doi: 10.1002/jlcr.3686. Epub 2018 Oct 25. J Labelled Comp Radiopharm. 2018. PMID: 30286517 Free PMC article.
-
[11C]Carbon monoxide: advances in production and application to PET radiotracer development over the past 15 years.EJNMMI Radiopharm Chem. 2019 Sep 18;4(1):25. doi: 10.1186/s41181-019-0073-4. EJNMMI Radiopharm Chem. 2019. PMID: 31659516 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources