Perimenopause and emergence of an Alzheimer's bioenergetic phenotype in brain and periphery
- PMID: 29016679
- PMCID: PMC5634623
- DOI: 10.1371/journal.pone.0185926
Perimenopause and emergence of an Alzheimer's bioenergetic phenotype in brain and periphery
Erratum in
-
Correction: Perimenopause and emergence of an Alzheimer's bioenergetic phenotype in brain and periphery.PLoS One. 2018 Feb 15;13(2):e0193314. doi: 10.1371/journal.pone.0193314. eCollection 2018. PLoS One. 2018. PMID: 29447296 Free PMC article.
Abstract
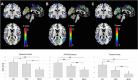
After advanced age, female sex is the major risk factor for Alzheimer's disease (AD). The biological mechanisms underlying the increased AD risk in women remain largely undetermined. Preclinical studies identified the perimenopause to menopause transition, a neuroendocrine transition state unique to the female, as a sex-specific risk factor for AD. In animals, estrogenic regulation of cerebral glucose metabolism (CMRglc) falters during perimenopause. This is evident in glucose hypometabolism and decline in mitochondrial efficiency which is sustained thereafter. This study bridges basic to clinical science to characterize brain bioenergetics in a cohort of forty-three, 40-60 year-old clinically and cognitively normal women at different endocrine transition stages including premenopause (controls, CNT, n = 15), perimenopause (PERI, n = 14) and postmenopause (MENO, n = 14). All participants received clinical, laboratory and neuropsychological examinations, 18F-fluoro-deoxyglucose (FDG)-Positron Emission Tomography (PET) FDG-PET scans to estimate CMRglc, and platelet mitochondrial cytochrome oxidase (COX) activity measures. Statistical parametric mapping and multiple regression models were used to examine clinical, CMRglc and COX data across groups. As expected, the MENO group was older than PERI and controls. Groups were otherwise comparable for clinical measures and distribution of APOE4 genotype. Both MENO and PERI groups exhibited reduced CMRglc in AD-vulnerable regions which was correlated with decline in mitochondrial COX activity compared to CNT (p's<0.001). A gradient in biomarker abnormalities was most pronounced in MENO, intermediate in PERI, and lowest in CNT (p<0.001). Biomarkers correlated with immediate and delayed memory scores (Pearson's 0.26≤r≤0.32, p≤0.05). These findings validate earlier preclinical findings and indicate emergence of bioenergetic deficits in perimenopausal and postmenopausal women, suggesting that the optimal window of opportunity for therapeutic intervention in women is early in the endocrine aging process.
Conflict of interest statement
Figures

References
-
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. Jama. 1997;278(16):1349–56. . - PubMed
-
- Alzheimer's A. 2016 Alzheimer's disease facts and figures. Alzheimer's & dementia: the journal of the Alzheimer's Association. 2016;12(4):459–509. . - PubMed
-
- Vina J, Lloret A. Why women have more Alzheimer's disease than men: gender and mitochondrial toxicity of amyloid-beta peptide. Journal of Alzheimer's disease: JAD. 2010;20 Suppl 2:S527–33. doi: 10.3233/JAD-2010-100501 . - DOI - PubMed
-
- Brinton RD, Yao J, Yin F, Mack WJ, Cadenas E. Perimenopause as a neurological transition state. Nature reviews Endocrinology. 2015;11(7):393–405. doi: 10.1038/nrendo.2015.82 . - DOI - PMC - PubMed
-
- Sperling RA, Karlawish J, Johnson KA. Preclinical Alzheimer disease-the challenges ahead. Nature reviews Neurology. 2013;9(1):54–8. doi: 10.1038/nrneurol.2012.241 ; PubMed Central PMCID: PMC3643203. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

