Investigation of cardiac fibroblasts using myocardial slices
- PMID: 29016704
- PMCID: PMC5852538
- DOI: 10.1093/cvr/cvx152
Investigation of cardiac fibroblasts using myocardial slices
Abstract
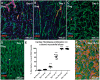
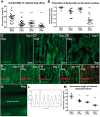
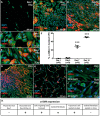
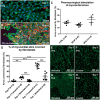
Aims: Cardiac fibroblasts (CFs) are considered the principal regulators of cardiac fibrosis. Factors that influence CF activity are difficult to determine. When isolated and cultured in vitro, CFs undergo rapid phenotypic changes including increased expression of α-SMA. Here we describe a new model to study CFs and their response to pharmacological and mechanical stimuli using in vitro cultured mouse, dog and human myocardial slices.
Methods and results: Unloading of myocardial slices induced CF proliferation without α-SMA expression up to 7 days in culture. CFs migrating onto the culture plastic support or cultured on glass expressed αSMA within 3 days. The cells on the slice remained αSMA(-) despite transforming growth factor-β (20 ng/ml) or angiotensin II (200 µM) stimulation. When diastolic load was applied to myocardial slices using A-shaped stretchers, CF proliferation was significantly prevented at Days 3 and 7 (P < 0.001).
Conclusions: Myocardial slices allow the study of CFs in a multicellular environment and may be used to effectively study mechanisms of cardiac fibrosis and potential targets.
Keywords: Cardiac fibroblasts; Fibrosis; Mechanical load; Myocardial slices; α-SMA.
© The Author 2017 Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures




References
-
- Abramochkin DV, Lozinsky IT, Kamkin A.. Influence of mechanical stress on fibroblast-myocyte interactions in mammalian heart. J Mol Cell Cardiol 2014;70:27–36. - PubMed
-
- Cartledge J. The Effects of Cardiac Fibroblasts on Cardiac Myocyte Structure and Excitation-Contraction Coupling Through Paracrine Mediators. National Heart and Lung Institute; 2013.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

