Differential eNOS-signalling by platelet subpopulations regulates adhesion and aggregation
- PMID: 29016749
- PMCID: PMC5852541
- DOI: 10.1093/cvr/cvx179
Differential eNOS-signalling by platelet subpopulations regulates adhesion and aggregation
Abstract
Aims: In addition to maintaining haemostasis, circulating blood platelets are the cellular culprits that form occlusive thrombi in arteries and veins. Compared to blood leucocytes, which exist as functionally distinct subtypes, platelets are considered to be relatively simple cell fragments that form vascular system plugs without a differentially regulated cellular response. Hence, investigation into platelet subpopulations with distinct functional roles in haemostasis/thrombosis has been limited. In our present study, we investigated whether functionally distinct platelet subpopulations exist based on their ability to generate and respond to nitric oxide (NO), an endogenous platelet inhibitor.
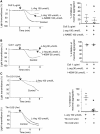
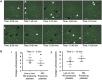
Methods and results: Utilizing highly sensitive and selective flow cytometry protocols, we demonstrate that human platelet subpopulations exist based on the presence and absence of endothelial nitric oxide synthase (eNOS). Platelets lacking eNOS (approximately 20% of total platelets) fail to produce NO and have a down-regulated soluble guanylate cyclase-protein kinase G (sGC-PKG)-signalling pathway. In flow chamber and aggregation experiments eNOS-negative platelets primarily initiate adhesion to collagen, more readily activate integrin αIIbβ3 and secrete matrix metalloproteinase-2, and form larger aggregates than their eNOS-positive counterparts. Conversely, platelets having an intact eNOS-sGC-PKG-signalling pathway (approximately 80% of total platelets) form the bulk of an aggregate via increased thromboxane synthesis and ultimately limit its size via NO generation.
Conclusion: These findings reveal previously unrecognized characteristics and complexity of platelets and their regulation of adhesion/aggregation. The identification of platelet subpopulations also has potentially important consequences to human health and disease as impaired platelet NO-signalling has been identified in patients with coronary artery disease.
Keywords: Aggregation; Endothelial nitric oxide synthase; Flow cytometry; Nitric oxide; Platelet subpopulations.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author 2017. For permissions please email: journals.permissions@oup.com.
Figures





References
-
- Rand ML, Greenberg JP, Packham MA, Mustard JF.. Density subpopulations of rabbit platelets: size, protein, and sialic acid content, and specific radioactivity changes following labeling with 35S-sulfate in vivo. Blood 1981;57:741–746. - PubMed
-
- Thompson CB, Eaton K, Princiotta SM, Rushin CA, Valeri CR.. Size dependent platelet subpopulations: relationship of platelet volume to ultrastructure, enzymatic activity, and function. Br J Haematol 1982;50:509–519. - PubMed
-
- Dale GL, Friese P, Batar P, Hamilton SF, Reed GL, Jackson KW, Clemetson KJ, Alberio L.. Stimulated platelets use serotonin to enhance their retention of procoagulant proteins on the cell surface. Nature 2002;415:175–179. - PubMed
-
- London FS, Marcinkiewicz M, Walsh PN.. A Subpopulation of platelets responds to thrombin- or SFLLRN-stimulation with binding sites for factor IXa. J Biol Chem 2004;279:19854–19859. - PubMed
-
- Mason KD, Carpinelli MR, Fletcher JI, Collinge JE, Hilton AA, Ellis S, Kelly PN, Ekert PG, Metcalf D, Roberts AW, Huang DCS, Kile BT.. Programmed anuclear cell death delimits platelet life span. Cell 2007;128:1173–1186. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

