A point mutation in the ion conduction pore of AMPA receptor GRIA3 causes dramatically perturbed sleep patterns as well as intellectual disability
- PMID: 29016847
- PMCID: PMC5639461
- DOI: 10.1093/hmg/ddx270
A point mutation in the ion conduction pore of AMPA receptor GRIA3 causes dramatically perturbed sleep patterns as well as intellectual disability
Abstract
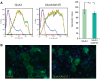
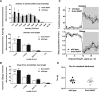
The discovery of genetic variants influencing sleep patterns can shed light on the physiological processes underlying sleep. As part of a large clinical sequencing project, WGS500, we sequenced a family in which the two male children had severe developmental delay and a dramatically disturbed sleep-wake cycle, with very long wake and sleep durations, reaching up to 106-h awake and 48-h asleep. The most likely causal variant identified was a novel missense variant in the X-linked GRIA3 gene, which has been implicated in intellectual disability. GRIA3 encodes GluA3, a subunit of AMPA-type ionotropic glutamate receptors (AMPARs). The mutation (A653T) falls within the highly conserved transmembrane domain of the ion channel gate, immediately adjacent to the analogous residue in the Grid2 (glutamate receptor) gene, which is mutated in the mouse neurobehavioral mutant, Lurcher. In vitro, the GRIA3(A653T) mutation stabilizes the channel in a closed conformation, in contrast to Lurcher. We introduced the orthologous mutation into a mouse strain by CRISPR-Cas9 mutagenesis and found that hemizygous mutants displayed significant differences in the structure of their activity and sleep compared to wild-type littermates. Typically, mice are polyphasic, exhibiting multiple sleep bouts of sleep several minutes long within a 24-h period. The Gria3A653T mouse showed significantly fewer brief bouts of activity and sleep than the wild-types. Furthermore, Gria3A653T mice showed enhanced period lengthening under constant light compared to wild-type mice, suggesting an increased sensitivity to light. Our results suggest a role for GluA3 channel activity in the regulation of sleep behavior in both mice and humans.
© The Author 2017. Published by Oxford University Press.
Figures






References
-
- Tobler I. (1995) Is sleep fundamentally different between mammalian species? Behav Brain Res., 69, 35–41. - PubMed
-
- Borbely A.A. (1982) A two process model of sleep regulation. Hum. Neurobiol., 1, 195–204. - PubMed
-
- Fisher S.P., Foster R.G., Peirson S.N. (2013) The circadian control of sleep. Handb. Exp. Pharmacol., 217, 157–183. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

