Maternal BMI at the start of pregnancy and offspring epigenome-wide DNA methylation: findings from the pregnancy and childhood epigenetics (PACE) consortium
- PMID: 29016858
- PMCID: PMC5656174
- DOI: 10.1093/hmg/ddx290
Maternal BMI at the start of pregnancy and offspring epigenome-wide DNA methylation: findings from the pregnancy and childhood epigenetics (PACE) consortium
Abstract
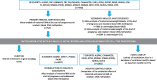
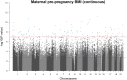
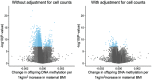
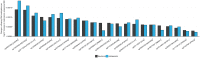
Pre-pregnancy maternal obesity is associated with adverse offspring outcomes at birth and later in life. Individual studies have shown that epigenetic modifications such as DNA methylation could contribute. Within the Pregnancy and Childhood Epigenetics (PACE) Consortium, we meta-analysed the association between pre-pregnancy maternal BMI and methylation at over 450,000 sites in newborn blood DNA, across 19 cohorts (9,340 mother-newborn pairs). We attempted to infer causality by comparing the effects of maternal versus paternal BMI and incorporating genetic variation. In four additional cohorts (1,817 mother-child pairs), we meta-analysed the association between maternal BMI at the start of pregnancy and blood methylation in adolescents. In newborns, maternal BMI was associated with small (<0.2% per BMI unit (1 kg/m2), P < 1.06 × 10-7) methylation variation at 9,044 sites throughout the genome. Adjustment for estimated cell proportions greatly attenuated the number of significant CpGs to 104, including 86 sites common to the unadjusted model. At 72/86 sites, the direction of the association was the same in newborns and adolescents, suggesting persistence of signals. However, we found evidence for acausal intrauterine effect of maternal BMI on newborn methylation at just 8/86 sites. In conclusion, this well-powered analysis identified robust associations between maternal adiposity and variations in newborn blood DNA methylation, but these small effects may be better explained by genetic or lifestyle factors than a causal intrauterine mechanism. This highlights the need for large-scale collaborative approaches and the application of causal inference techniques in epigenetic epidemiology.
© The Author 2017. Published by Oxford University Press.
Figures

References
-
- Gaillard R., Steegers E.A.P., Duijts L., Felix J.F., Hofman A., Franco O.H., Jaddoe V.W.V. (2014) Childhood cardiometabolic outcomes of maternal obesity during pregnancy: the Generation R Study. Hypertension, 63, 683–691. - PubMed
-
- Van Lieshout R.J., Taylor V.H., Boyle M.H. (2011) Pre-pregnancy and pregnancy obesity and neurodevelopmental outcomes in offspring: a systematic review. Obes. Rev., 12, e548–e559. - PubMed
Publication types
MeSH terms
Grants and funding
- R24 ES028507/ES/NIEHS NIH HHS/United States
- R01 MH094609/MH/NIMH NIH HHS/United States
- R01 ES021369/ES/NIEHS NIH HHS/United States
- MC_PC_15018/MRC_/Medical Research Council/United Kingdom
- P01 ES018172/ES/NIEHS NIH HHS/United States
- R01 DK103246/DK/NIDDK NIH HHS/United States
- P01 ES022832/ES/NIEHS NIH HHS/United States
- K12 HD043446/HD/NICHD NIH HHS/United States
- R25 CA134286/CA/NCI NIH HHS/United States
- 648916/ERC_/European Research Council/International
- R01 AI121226/AI/NIAID NIH HHS/United States
- R01 ES022223/ES/NIEHS NIH HHS/United States
- MC_UU_12013/1/MRC_/Medical Research Council/United Kingdom
- P50 ES018172/ES/NIEHS NIH HHS/United States
- R01 ES025531/ES/NIEHS NIH HHS/United States
- MC_UU_12013/5/MRC_/Medical Research Council/United Kingdom
- 001/WHO_/World Health Organization/International
- G9815508/MRC_/Medical Research Council/United Kingdom
- P20 GM104416/GM/NIGMS NIH HHS/United States
- MC_UU_12013/2/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

