YUCCA9-Mediated Auxin Biosynthesis and Polar Auxin Transport Synergistically Regulate Regeneration of Root Systems Following Root Cutting
- PMID: 29016906
- PMCID: PMC5921505
- DOI: 10.1093/pcp/pcx107
YUCCA9-Mediated Auxin Biosynthesis and Polar Auxin Transport Synergistically Regulate Regeneration of Root Systems Following Root Cutting
Abstract
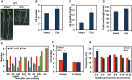
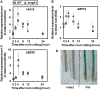
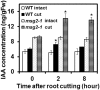
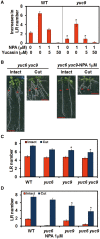
Recovery of the root system following physical damage is an essential issue for plant survival. An injured root system is able to regenerate by increases in lateral root (LR) number and acceleration of root growth. The horticultural technique of root pruning (root cutting) is an application of this response and is a common garden technique for controlling plant growth. Although root pruning is widely used, the molecular mechanisms underlying the subsequent changes in the root system are poorly understood. In this study, root pruning was employed as a model system to study the molecular mechanisms of root system regeneration. Notably, LR defects in wild-type plants treated with inhibitors of polar auxin transport (PAT) or in the auxin signaling mutant auxin/indole-3-acetic acid19/massugu2 were recovered by root pruning. Induction of IAA19 following root pruning indicates an enhancement of auxin signaling by root pruning. Endogenous levels of IAA increased after root pruning, and YUCCA9 was identified as the primary gene responsible. PAT-related genes were induced after root pruning, and the YUCCA inhibitor yucasin suppressed root regeneration in PAT-related mutants. Therefore, we demonstrate the crucial role of YUCCA9, along with other redundant YUCCA family genes, in the enhancement of auxin biosynthesis following root pruning. This further enhances auxin transport and activates downstream auxin signaling genes, and thus increases LR number.
Keywords: Auxin biosynthesis; Lateral root; Polar auxin transport; Root pruning; YUCCA9; Yucasin.
© The Author 2017. Published by Oxford University Press on behalf of Japanese Society of Plant Physiologists.
Figures





References
-
- Al-Ghazi Y., Muller B., Pinloche S., Tranbarger T.J., Nacry P., Rossignol M.. et al. (2003) Temporal responses of Arabidopsis root architecture to phosphate starvation: evidence for the involvement of auxin signaling. Plant Cell Environ. 26: 1053–1066.
-
- Beeckman T., Burssens S., Inzé D. (2001) The peri-cell-cycle in Arabidopsis. J. Exp. Bot. 52: 403–411. - PubMed
-
- Benková E., Michniewicz M., Sauer M., Teichmann T., Seifertová D., Jürgens G.. et al. (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602. - PubMed
-
- Biddington N.L., Dearman A.S. (1984) Shoot and root growth of lettuce seedlings following root pruning. Ann. Bot. 53: 663–668.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

