Early planned removal of umbilical venous catheters to prevent infection in newborn infants
- PMID: 29017005
- PMCID: PMC6485860
- DOI: 10.1002/14651858.CD012142.pub2
Early planned removal of umbilical venous catheters to prevent infection in newborn infants
Abstract
Background: Lengthy duration of use may be a risk factor for umbilical venous catheter-associated bloodstream infection in newborn infants. Early planned removal of umbilical venous catheters (UVCs) is recommended to reduce the incidence of infection and associated morbidity and mortality.
Objectives: To compare the effectiveness of early planned removal of UVCs (up to two weeks after insertion) versus an expectant approach or a longer fixed duration in preventing bloodstream infection and other complications in newborn infants.To perform subgroup analyses by gestational age at birth and prespecified planned duration of UVC placement (see "Subgroup analysis and investigation of heterogeneity").
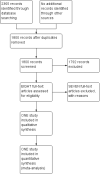
Search methods: We used the standard Cochrane Neonatal search strategy including electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 4), Ovid MEDLINE, Embase, and the Maternity & Infant Care Database (until May 2017), as well as conference proceedings and previous reviews.
Selection criteria: Randomised and quasi-randomised controlled trials that compared effects of early planned removal of UVCs (up to two weeks after insertion) versus an expectant approach or a longer fixed duration in preventing bloodstream infection and other complications in newborn infants.
Data collection and analysis: Two review authors assessed trial eligibility and risk of bias and independently undertook data extraction. We analysed treatment effects and reported risk ratio (RR) and risk difference (RD) for dichotomous data, and mean difference (MD) for continuous data, with respective 95% confidence intervals (CIs). We planned to use a fixed-effect model in meta-analyses and to explore potential causes of heterogeneity in sensitivity analyses. We assessed the quality of evidence for the main comparison at the outcome level using GRADE methods.
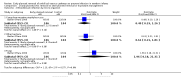
Main results: We found one eligible trial. Participants were 210 newborn infants with birth weight less than 1251 grams. The trial was unblinded but otherwise of good methodological quality. This trial compared removal of an umbilical venous catheter within 10 days after insertion (and replacement with a peripheral cannula or a percutaneously inserted central catheter as required) versus expectant management (UVC in place up to 28 days). More infants in the early planned removal group than in the expectant management group (83 vs 33) required percutaneous insertion of a central catheter (PICC). Trial results showed no difference in the incidence of catheter-related bloodstream infection (RR 0.65, 95% CI 0.35 to 1.22), in hospital mortality (RR 1.12, 95% CI 0.42 to 2.98), in catheter-associated thrombus necessitating removal (RR 0.33, 95% confidence interval 0.01 to 7.94), or in other morbidity. GRADE assessment indicated that the quality of evidence was "low" at outcome level principally as the result of imprecision and risk of surveillance bias due to lack of blinding in the included trial.
Authors' conclusions: Currently available trial data are insufficient to show whether early planned removal of umbilical venous catheters reduces risk of infection, mortality, or other morbidity in newborn infants. A large, simple, and pragmatic randomised controlled trial is needed to resolve this ongoing uncertainty.
Conflict of interest statement
None of the review authors reports a potential conflict of interest.
Figures
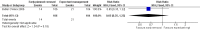




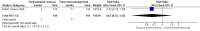






Update of
References
References to studies included in this review
Butler O'Hara 2006 {published and unpublished data}
-
- Butler‐O'Hara M, Buzzard.CJ, Reubens L, McDermott MP, DiGrazio W, D'Angio CT. A randomized trial comparing long‐term and short‐term use of umbilical venous catheters in premature infants with birth weights of less than 1251 grams. Pediatrics 2006;118(1):e25‐35. [DOI: 10.1542/peds.2005-1880; PUBMED: 16785289] - DOI - PubMed
References to studies excluded from this review
Boo 1999 {published data only}
-
- Boo NY, Wong NC, Zulkifli SS, Lye MS. Risk factors associated with umbilical vascular catheter‐associated thrombosis in newborn infants. Journal of Paediatrics and Child Health 1999;35(5):460‐5. [PUBMED: 10571759] - PubMed
Davey 1994 {published data only}
-
- Davey AM, Wagner CL, Cox C, Kendig JW. Feeding premature infants while low umbilical artery catheters are in place: a prospective, randomized trial. Journal of Pediatrics 1994;124(5 Pt 1):795‐9. [PUBMED: 8176571] - PubMed
Gharehbaghi 2011 {published data only}
Keir 2014 {published data only}
-
- Keir A, Giesinger R, Dunn M. How long should umbilical venous catheters remain in place in neonates who require long‐term (>/=5‐7 days) central venous access?. Journal of Paediatrics and Child Health 2014;50(8):649‐52. [PUBMED: 25080979] - PubMed
Khilnani 1991 {published data only}
-
- Khilnani P, Goldstein B, Todres ID. Double lumen umbilical venous catheters in critically ill neonates: a randomized prospective study. Critical Care Medicine 1991;19(11):1348‐51. [PUBMED: 1935151] - PubMed
Landers 1991 {published data only}
-
- Landers S, Moise AA, Fraley JK, Smith EO, Baker CJ. Factors associated with umbilical catheter‐related sepsis in neonates. American Journal of Diseases of Children 1991;145(6):675‐80. [PUBMED: 1903588] - PubMed
Loisel 1996 {published data only}
-
- Loisel DB, Smith MM, MacDonald MG, Martin GR. Intravenous access in newborn infants: impact of extended umbilical venous catheter use on requirement for peripheral venous lines. Journal of Perinatology 1996;16(6):461‐6. [PUBMED: 8979185] - PubMed
Additional references
Adams‐Chapman 2006
Arnts 2014
Balain 2015
Bassler 2009
-
- Bassler D, Stoll BJ, Schmidt B, Asztalos EV, Roberts RS, Robertson CM, et al. Trial of Indomethacin Prophylaxis in Preterms Investigators. Using a count of neonatal morbidities to predict poor outcome in extremely low birth weight infants: added role of neonatal infection. Pediatrics 2009;123(1):313‐8. [DOI: 10.1542/peds.2008-0377; PUBMED: 19117897] - DOI - PMC - PubMed
Butler‐O'Hara 2012
Chapman 2003
Ehrenkranz 2005
-
- Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, et al. National Institutes of Child Health and Human Development Neonatal Research Network. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics 2005;116(6):1353‐60. [DOI: 10.1542/peds.2005-0249; PUBMED: 16322158] - DOI - PubMed
Gordon 2016
GRADEproGDT [Computer program]
-
- McMaster University (developed by Evidence Prime). GRADEproGDT. Version accessed 10 May 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. gradepro.org.
Green 1998
-
- Green C, Yohannan MD. Umbilical arterial and venous catheters: placement, use, and complications. Neonatal Network 1998;17(6):23‐8. [PUBMED: 9832755] - PubMed
Grizelj 2014
Guyatt 2011a
-
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] - PubMed
Guyatt 2011b
-
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence ‐ study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407‐15. [PUBMED: 21247734] - PubMed
Guyatt 2011c
-
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence ‐ inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [PUBMED: 21803546] - PubMed
Guyatt 2011d
-
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence ‐ imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] - PubMed
Guyatt 2011e
-
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence ‐ indirectness. Journal of Clinical Epidemiology 2011;64(12):1303‐10. [PUBMED: 21802903] - PubMed
Hermans 2007
-
- Hermans D, Talbotec C, Lacaille F, Goulet O, Ricour C, Colomb V. Early central catheter infections may contribute to hepatic fibrosis in children receiving long‐term parenteral nutrition. Journal of Pediatric Gastroenterology and Nutrition 2007;44(4):459‐63. [DOI: 10.1097/MPG.0b013e318031a5c7; PUBMED: 17414144] - DOI - PubMed
Hermansen 2005
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. handbook.cochrane.org.
Hollingsworth 2015
-
- Hollingsworth C, Clarke P, Sharma A, Upton M. National survey of umbilical venous catheterisation practices in the wake of two deaths. Archives of Disease in Childhood. Fetal and Neonatal Edition 2015; Vol. 100, issue 4:F371‐2. [DOI: 10.1136/archdischild-2015-308327; PUBMED: 25862724] - DOI - PubMed
ICROP 2005
Kim 2001
Lahra 2009
Mutlu 2016
Narang 2009
Nash 2006
-
- Nash P. Umbilical catheters, placement, and complication management. Journal of Infusion Nursing 2006;29(6):346‐52. [PUBMED: 17122690] - PubMed
O'Grady 2011
-
- O'Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, et al. Healthcare Infection Control Practices Advisory Committee (HICPAC). Guidelines for the prevention of intravascular catheter‐related infections. Clinical Infectious Diseases 2011;52(9):e162‐93. [DOI: 10.1093/cid/cir257; PUBMED: 21460264] - DOI - PMC - PubMed
Panetta 2000
-
- Panetta J, Morley C, Betheras R. Ascites in a premature baby due to parenteral nutrition from an umbilical venous catheter. Journal of Paediatrics and Child Health 2000;36(2):197‐8. [PUBMED: 10809532] - PubMed
Payne 2004
-
- Payne NR, Carpenter JH, Badger GJ, Horbar JD, Rogowski J. Marginal increase in cost and excess length of stay associated with nosocomial bloodstream infections in surviving very low birth weight infants. Pediatrics 2004;114(2):348‐55. [PUBMED: 15286215] - PubMed
Pereira 1992
RevMan 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Saint 2000
Schulman 2011
-
- Schulman J, Stricof R, Stevens TP, Horgan M, Gase K, Holzman IR, et al. New York State Regional Perinatal Care Centers. Statewide NICU central‐line‐associated bloodstream infection rates decline after bundles and checklists. Pediatrics 2011;127(3):436‐44. [DOI: 10.1542/peds.2010-2873; PUBMED: 21339265] - DOI - PubMed
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE Working Group. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. https://gdt.gradepro.org/app/handbook/handbook.html. Updated October 2013.
Seguin 1994
Shah 2008
-
- Shah DK, Doyle LW, Anderson PJ, Bear M, Daley AJ, Hunt RW, et al. Adverse neurodevelopment in preterm infants with postnatal sepsis or necrotizing enterocolitis is mediated by white matter abnormalities on magnetic resonance imaging at term. Journal of Pediatrics 2008;153(2):170‐5. [DOI: 10.1016/j.jpeds.2008.02.033; PUBMED: 18534228] - DOI - PubMed
Shahid 2014
Shalabi 2015
Shareena 2008
-
- Shareena I, Khu YS, Cheah FC. Intraperitoneal extravasation of total parenteral nutrition infusate from an umbilical venous catheter. Singapore Medical Journal 2008;49(2):e35‐6. [PUBMED: 18301823] - PubMed
Stoll 2004
-
- Stoll BJ, Hansen NI, Adams‐Chapman I, Fanaroff AA, Hintz SR, Vohr B, et al. National Institute of Child Health and Human Development Neonatal Research Network. Neurodevelopmental and growth impairment among extremely low‐birth‐weight infants with neonatal infection. JAMA 2004;292(19):2357‐65. [DOI: 10.1001/jama.292.19.2357; PUBMED: 15547163] - DOI - PubMed
Taylor 2014
Taylor 2015
Traen 2005
Walsh 1986
Wu 2012
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

