Fgf10-Hippo Epithelial-Mesenchymal Crosstalk Maintains and Recruits Lung Basal Stem Cells
- PMID: 29017029
- PMCID: PMC5679744
- DOI: 10.1016/j.devcel.2017.09.003
Fgf10-Hippo Epithelial-Mesenchymal Crosstalk Maintains and Recruits Lung Basal Stem Cells
Abstract
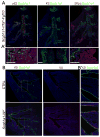
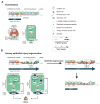
The lung harbors its basal stem/progenitor cells (BSCs) in the protected environment of the cartilaginous airways. After major lung injuries, BSCs are activated and recruited to sites of injury. Here, we show that during homeostasis, BSCs in cartilaginous airways maintain their stem cell state by downregulating the Hippo pathway (resulting in increased nuclear Yap), which generates a localized Fgf10-expressing stromal niche; in contrast, differentiated epithelial cells in non-cartilaginous airways maintain quiescence by activating the Hippo pathway and inhibiting Fgf10 expression in airway smooth muscle cells (ASMCs). However, upon injury, surviving differentiated epithelial cells spread to maintain barrier function and recruit integrin-linked kinase to adhesion sites, which leads to Merlin degradation, downregulation of the Hippo pathway, nuclear Yap translocation, and expression and secretion of Wnt7b. Epithelial-derived Wnt7b, then in turn, induces Fgf10 expression in ASMCs, which extends the BSC niche to promote regeneration.
Keywords: Fgf10; Fgfr2; Hippo; Ilk; Yap; basal stem cell; integrin; lung; regeneration; stem cell niche.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflict of interest
Figures





References
-
- Auerbach O, Stout AP, Hammond EC, Garfinkel L. Changes in bronchial epithelium in relation to cigarette smoking and in relation to lung cancer. N Engl J Med. 1961;265:253–267. - PubMed
-
- Bellusci S, Grindley J, Emoto H, Itoh N, Hogan BL. Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development. 1997;124:4867. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

