Patiromer Lowers Serum Potassium When Taken without Food: Comparison to Dosing with Food from an Open-Label, Randomized, Parallel Group Hyperkalemia Study
- PMID: 29017162
- PMCID: PMC5804834
- DOI: 10.1159/000481270
Patiromer Lowers Serum Potassium When Taken without Food: Comparison to Dosing with Food from an Open-Label, Randomized, Parallel Group Hyperkalemia Study
Abstract
Background: Patiromer is a sodium-free, nonabsorbed, potassium binder approved for treatment of hyperkalemia. This open-label study compares the efficacy and safety of patiromer administered without food versus with food.
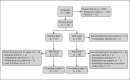
Methods: Adults with hyperkalemia (potassium ≥5.0 mEq/L) were randomized (1:1) to receive patiromer once daily without food or with food for 4 weeks. The dosage was adjusted (maximum: 25.2 g/day) using a prespecified titration schedule to achieve and maintain potassium within a target range (3.8-5.0 mEq/L). The primary efficacy endpoint was the proportion of patients with serum potassium in the target range at either week 3 or week 4. Safety was assessed by adverse events (AEs) and laboratory testing.
Results: Efficacy was evaluated in 112 patients; 65.2% were ≥65 years of age, 75.9% had chronic kidney disease, and 82.1% had diabetes. Baseline mean serum potassium was similar in the without-food (5.44 mEq/L) and with-food (5.34 mEq/L) groups. The primary endpoint was achieved by 87.3% (95% CI 75.5-94.7) and 82.5% (95% CI 70.1-91.3) of patients in the with-food and without-food groups, respectively; least squares mean changes in serum potassium from baseline to week 4 were -0.65 and -0.62 mEq/L, respectively (p < 0.0001). The most common AEs were diarrhea and constipation. Serum K+ remained ≥3.5 mEq/L in all patients; 5 patients developed serum magnesium <1.4 mg/dL, including 4 whose baseline magnesium was below the lower limit of normal.
Conclusion: Patiromer is equally effective and well tolerated when taken without food or with food, thereby offering the potential for dosing flexibility.
Keywords: Hyperkalemia; Patiromer; Potassium.
© 2017 The Author(s) Published by S. Karger AG, Basel.
Figures



References
-
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL, American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines 2013 ACCF/AHA guideline for the management of heart failure: a report of the American college of cardiology foundation/American heart association task force on practice guidelines. Circulation. 2013;128:e240–e327. - PubMed
-
- National Kidney Foundation . NKF KDOQI Guidelines: K/DOQI Clinical Practice Guidelines on Hypertension and Antihypertensive Agents in Chronic Kidney Disease. New York: National Kidney Foundation; 2004. http://www2.kidney.org/professionals/KDOQI/guidelines_bp/guide_11.htm (accessed June 6, 2017).
-
- Garg R, Yusuf S. Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. JAMA. 1995;273:1450–1456. - PubMed
-
- Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709–717. - PubMed
-
- Cohn JN, Tognoni G, Valsartan Heart Failure Trial Investigators A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667–1675. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

