Multiplex PCR point of care testing versus routine, laboratory-based testing in the treatment of adults with respiratory tract infections: a quasi-randomised study assessing impact on length of stay and antimicrobial use
- PMID: 29017451
- PMCID: PMC5635493
- DOI: 10.1186/s12879-017-2784-z
Multiplex PCR point of care testing versus routine, laboratory-based testing in the treatment of adults with respiratory tract infections: a quasi-randomised study assessing impact on length of stay and antimicrobial use
Abstract
Background: Laboratory-based respiratory pathogen (RP) results are often available too late to influence clinical decisions such as hospitalisation or antibiotic treatment due to time delay in transport of specimens and testing schedules. Ward-based i.e. point of care (POC) testing providing rapid results may alter the clinical management pathway.
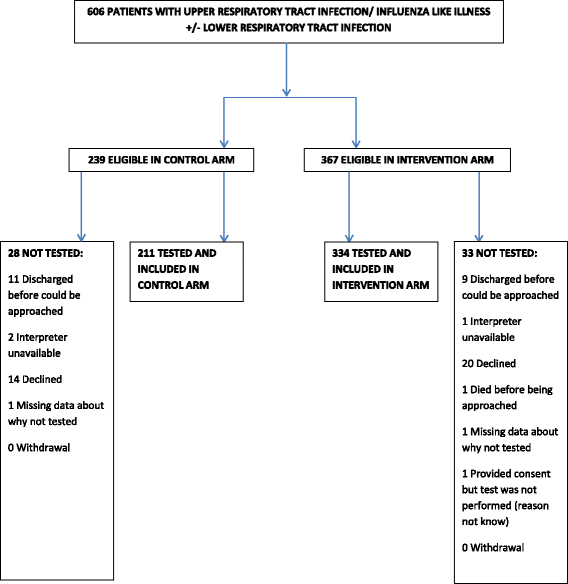
Methods: FilmArray® RP polymerase chain reaction (PCR) systems were placed in three in-patient and out-patient medical areas. Patients presenting with influenza-like illness /upper respiratory tract infection +/- lower RTI were recruited between January-July 2015. FilmArray® POC testing occurred on even days of the month (intervention) or routine, laboratory-based RP PCR testing +/- atypical serology on odd days (control). The primary outcome was length of hospital stay. The secondary outcomes were impact on the use of antimicrobials, readmissions, all-cause mortality, length of ward stay and turn-around time (TAT) (time to result from admission).
Results: Of 606 eligible patients, 545 (89.9%) were included; 211 in the control arm and 334 in the intervention arm. 20% of control arm patients and 24% of intervention arm patients had an RP detected. POC testing was not associated with the primary outcome measure, length of stay, but reduced the TAT from 39.5 h to 19.0 h, p < 0.001. Only the prescribing decision differed between study arms, p < 0.001. When antivirals were given, the intervention was associated with a reduction in the median time to the first dose of 36 h and allowed appropriate treatment of mycoplasma infection.
Conclusions: We found no association between respiratory PCR POC testing and length of stay or most of the secondary outcomes except the antimicrobial prescribing decision. This was probably due to a delay in initiating FilmArray® testing. Despite this, POC testing allowed time-critical antivirals to be given significantly faster, appropriate mycoplasma treatment and results were available considerably faster than routine, laboratory-based testing. Ward-staff of all grades performed POC testing without difficulty suggesting potential use across many divergent healthcare settings. Further studies evaluating the implementation of rapid respiratory PCR POC testing and the effect on length of stay and antimicrobial use are required.
Trial registration: ISRCTN10470967 , Retrospectively Registered, 30/6/2015.
Keywords: Adults; Antimicrobial stewardship; FilmArray®; Length of stay; Multiplex PCR; Point of care; Respiratory pathogens; Respiratory tract infection; Respiratory viruses.
Conflict of interest statement
Ethics approval and consent to participate
Written informed consent was obtained from each patient prior to participation in the study. Ethical approval for the study to proceed was obtained from the NRES Committee London – Westminster. REC reference 14/LO/1703.
Consent for publication
Not applicable
Competing interests
DJ has received speaker’s fees from bioMérieux.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Surveillance of influenza and other respiratory viruses, including novel respiratory viruses, in the United Kingdom: winter 2012/13, Public Health England. http://webarchive.nationalarchives.gov.uk/20140714084352/http://www.hpa.... Accessed on 15 Mar 2017.
-
- Musher DM, Thorner AR. Community-acquired pneumonia. N Engl J Med. 2015;372(3):294. - PubMed
-
- Lim WS, Baudouin SV, George RC, Hill AT, Jamieson C, Le Jeune I, Macfarlane JT, Read RC, Roberts HJ, Levy ML, Wani M, Woodhead MA. Guidelines for the Management of Community Acquired Pneumonia in adults: update 2009. Thorax 2009. Volume 64. Supplement III. - PubMed
-
- Muthuri SG, Venkatesan S, Myles PR, Leonardi-Bee J, Al Khuwaitir TS, Al Mamun A, et al. PRIDE Consortium Investigators. Effectiveness of neuraminidase inhibitors in reducing mortality in patients admitted to hospital with influenza a H1N1pdm09 virus infection: a meta-analysis of individual participant data. Lancet Respir Med. 2014;2(5):395–404. doi: 10.1016/S2213-2600(14)70041-4. - DOI - PMC - PubMed
-
- WHO Antimicrobial resistance fact sheet http://www.who.int/mediacentre/factsheets/fs194/en/ Accessed on 14 Dec 2016.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources