HGF potentiates extracellular matrix-driven migration of human myoblasts: involvement of matrix metalloproteinases and MAPK/ERK pathway
- PMID: 29017538
- PMCID: PMC5635537
- DOI: 10.1186/s13395-017-0138-6
HGF potentiates extracellular matrix-driven migration of human myoblasts: involvement of matrix metalloproteinases and MAPK/ERK pathway
Abstract
Background: The hepatocyte growth factor (HGF) is required for the activation of muscle progenitor cells called satellite cells (SC), plays a role in the migration of proliferating SC (myoblasts), and is present as a soluble factor during muscle regeneration, along with extracellular matrix (ECM) molecules. In this study, we aimed at determining whether HGF is able to interact with ECM proteins, particularly laminin 111 and fibronectin, and to modulate human myoblast migration.
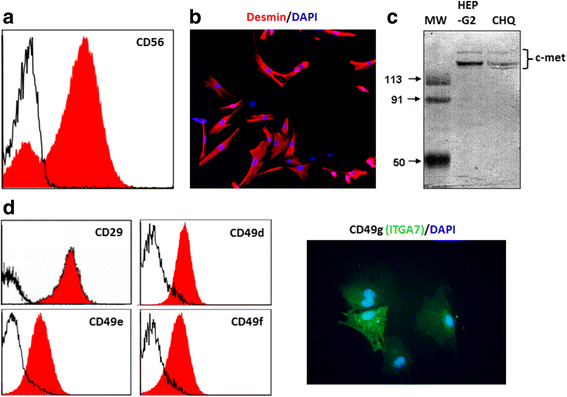
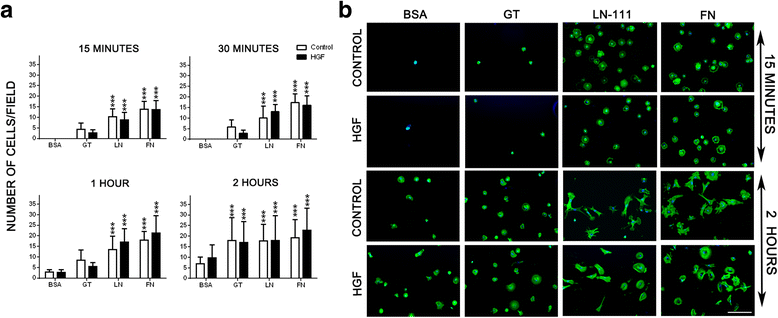
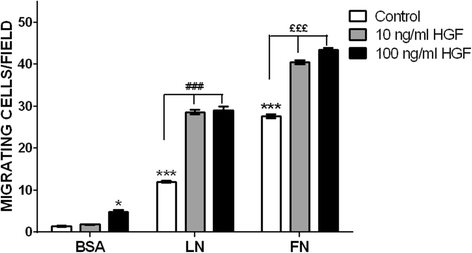
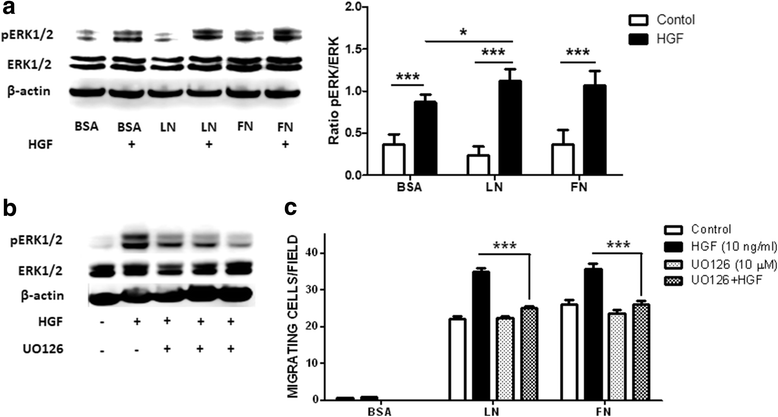
Methods: We evaluated the expression of the HGF-receptor c-Met, laminin, and fibronectin receptors by immunoblotting, flow cytometry, or immunofluorescence and used Transwell assays to analyze myoblast migration on laminin 111 and fibronectin in the absence or presence of HGF. Zymography was used to check whether HGF could modulate the production of matrix metalloproteinases by human myoblasts, and the activation of MAPK/ERK pathways was evaluated by immunoblotting.
Results: We demonstrated that human myoblasts express c-Met, together with laminin and fibronectin receptors. We observed that human laminin 111 and fibronectin have a chemotactic effect on myoblast migration, and this was synergistically increased when low doses of HGF were added. We detected an increase in MMP-2 activity in myoblasts treated with HGF. Conversely, MMP-2 inhibition decreased the HGF-associated stimulation of cell migration triggered by laminin or fibronectin. HGF treatment also induced in human myoblasts activation of MAPK/ERK pathways, whose specific inhibition decreased the HGF-associated stimulus of cell migration triggered by laminin 111 or fibronectin.
Conclusions: We demonstrate that HGF induces ERK phosphorylation and MMP production, thus stimulating human myoblast migration on ECM molecules. Conceptually, these data state that the mechanisms involved in the migration of human myoblasts comprise both soluble and insoluble moieties. This should be taken into account to optimize the design of therapeutic cell transplantation strategies by improving the migration of donor cells within the host tissue, a main issue regarding this approach.
Keywords: Fibronectin; HGF; Laminin; MAPK/ERK; Matrix metalloproteinases; Migration; Myoblast transplantation.
Conflict of interest statement
Ethics approval and consent to participate
The human myoblasts cells were obtained in accordance with French legislation on ethical rules (authorization AC-2013-1868 given by the bioethics committee of the French ministry).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

