Recommendations for reporting of systematic reviews and meta-analyses of diagnostic test accuracy: a systematic review
- PMID: 29017574
- PMCID: PMC5633882
- DOI: 10.1186/s13643-017-0590-8
Recommendations for reporting of systematic reviews and meta-analyses of diagnostic test accuracy: a systematic review
Abstract
Background: This study is to perform a systematic review of existing guidance on quality of reporting and methodology for systematic reviews of diagnostic test accuracy (DTA) in order to compile a list of potential items that might be included in a reporting guideline for such reviews: Preferred Reporting Items for Systematic Reviews and Meta-Analyses of Diagnostic Test Accuracy (PRISMA-DTA).
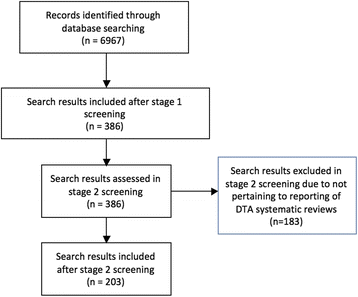
Methods: Study protocol published on EQUATOR website. Articles in full text or abstract form that reported on any aspect of reporting systematic reviews of diagnostic test accuracy were eligible for inclusion. We used the Ovid platform to search Ovid MEDLINE®, Ovid MEDLINE® In-Process & Other Non-Indexed Citations and Embase Classic+Embase through May 5, 2016. The Cochrane Methodology Register in the Cochrane Library (Wiley version) was also searched. Title and abstract screening followed by full-text screening of all search results was performed independently by two investigators. Guideline organization websites, published guidance statements, and the Cochrane Handbook for Diagnostic Test Accuracy were also searched. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and Standards for Reporting Diagnostic Accuracy (STARD) were assessed independently by two investigators for relevant items.
Results: The literature searched yielded 6967 results; 386 were included after title and abstract screening and 203 after full-text screening. After reviewing the existing literature and guidance documents, a preliminary list of 64 items was compiled into the following categories: title (three items); introduction (two items); methods (35 items); results (13 items); discussion (nine items), and disclosure (two items).
Conclusion: Items on the methods and reporting of DTA systematic reviews in the present systematic review will provide a basis for generating a PRISMA extension for DTA systematic reviews.
Conflict of interest statement
Ethics approval and consent to participate
Ethical approval is not required for this type of study at the authors’ institutions.
Consent for publication
All authors provide consent for publication.
Competing interests
David Moher is Editor-in-Chief of
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
-
- Institute of Medicine (US) Committee on Standards for Developing Trustworthy Clinical Practice Guidelines. Clinical Practice Guidelines We Can Trust. National Academies Press (US); 2011. https://www.ncbi.nlm.nih.gov/books/NBK209539/. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources