Family Integrated Care (FICare) in Level II Neonatal Intensive Care Units: study protocol for a cluster randomized controlled trial
- PMID: 29017578
- PMCID: PMC5634877
- DOI: 10.1186/s13063-017-2181-3
Family Integrated Care (FICare) in Level II Neonatal Intensive Care Units: study protocol for a cluster randomized controlled trial
Erratum in
-
Correction to: Family Integrated Care (FICare) in Level II Neonatal Intensive Care Units: study protocol for a cluster randomized controlled trial.Trials. 2020 Mar 19;21(1):282. doi: 10.1186/s13063-020-04246-w. Trials. 2020. PMID: 32192515 Free PMC article.
Abstract
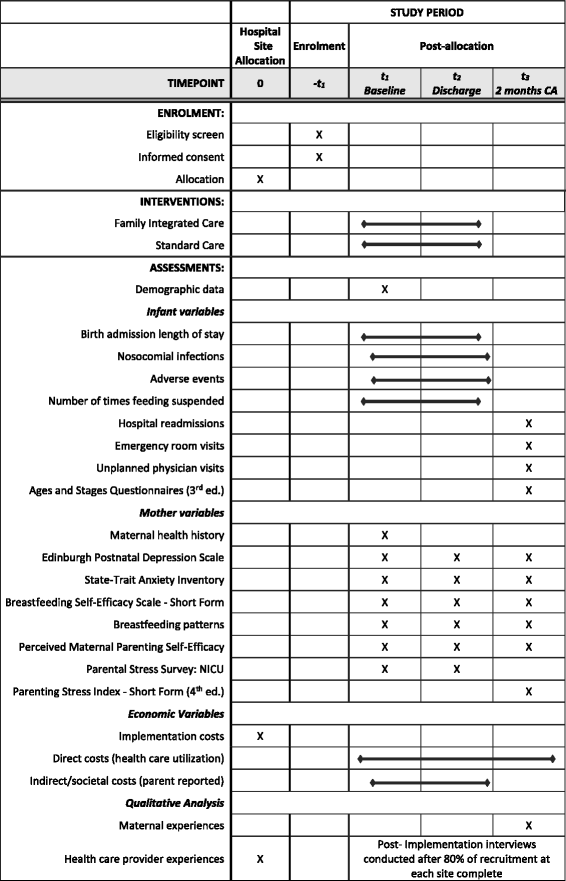
Background: Every year, about 15 million of the world's infants are born preterm (before 37 weeks gestation). In Alberta, the preterm birth rate was 8.7% in 2015, the second highest among Canadian provinces. Approximately 20% of preterm infants are born before 32 weeks gestation (early preterm), and require care in a Level III neonatal intensive care unit (NICU); 80% are born moderate (32 weeks and zero days [320/7] to 336/7 weeks) and late preterm (340/7 to 366/7 weeks), and require care in a Level II NICU. Preterm birth and experiences in the NICU disrupt early parent-infant relationships and induce parental psychosocial distress. Family Integrated Care (FICare) shows promise as a model of care in Level III NICUs. The purpose of this study is to evaluate length of stay, infant and maternal clinical outcomes, and costs following adaptation and implementation of FICare in Level II NICUs.
Methods: We will conduct a pragmatic, cluster randomized controlled trial (cRCT) in ten Alberta Level II NICUs allocated to one of two groups: FICare or standard care. The FICare Alberta model involves three theoretically-based, standardized components: information sharing, parenting education, and family support. Our sample size of 181 mother-infant dyads per group is based on the primary outcome of NICU length of stay, 80% participation, and 80% retention at follow-up. Secondary outcomes (e.g., infant clinical outcomes and maternal psychosocial distress) will be assessed shortly after admission to NICU, at discharge and 2 months corrected age. We will conduct economic analysis from two perspectives: the public healthcare payer and society. To understand the utility, acceptability, and impact of FICare, qualitative interviews will be conducted with a subset of mothers at the 2-month follow-up, and with hospital administrators and healthcare providers near the end of the study.
Discussion: Results of this pragmatic cRCT of FICare in Alberta Level II NICUs will inform policy decisions by providing evidence about the clinical effectiveness and costs of FICare.
Trial registration: ClinicalTrials.gov, ID: NCT02879799 . Registered on 27 May 2016. Protocol version: 9 June 2016; version 2.
Keywords: Cost-effectiveness; Family integrated care; Infant; Nursing; Parenting education; Patient engagement; Premature; Randomized controlled trial.
Conflict of interest statement
Authors’ information
KB (PhD, RN) is a Professor in the Faculty of Nursing and Adjunct Research Professor in the Department of Pediatrics, University of Calgary. VS (MSc, MD) is an academic Neonatologist at Mount Sinai Hospital, Toronto, and an Associate Professor in the Department of Paediatrics and Institute of Health Policy, Management and Evaluation, University of Toronto. She is currently conducting a study of FICare in Level II NICUs in Ontario. KA (MEd, MBBS, MA, MD) is a Staff Neonatologist, Edmonton Neonatal Program, and a Professor in the Department of Pediatrics, University of Alberta. WI (PhD) is a Research Associate and Health Economist, Saint Michael’s Hospital, Toronto. LP-D (PhD) is a Biostatistician and Adjunct Assistant Professor, University of Calgary. JS (MN, NNP) is a Neonatal Nurse Practitioner, Rockyview General Hospital, Calgary. JL (MN, NNP) is a Neonatal Nurse Practitioner, Royal Alexandra Hospital, Edmonton. KM is a Knowledge Translation Scientist, Alberta Health Services. ES (PhD) is an Adjunct Professor in the Faculty of Social Work, University of Calgary. CN (MSc, MD) is the Chief Pathologist, Calgary Lab Services. RC (MD) is the Chair of the Department of Obstetrics and Gynecology, University of Alberta. TS (PhD, MD) is an Associate Professor, University of Calgary, and Scientific Director of the Critical Care Strategic Clinical Network, Alberta Health Services. AL (MSc, MD, MBBS) is a Staff Neonatologist at Foothills Medical Centre, Calgary, and an Associate Professor in the Department of Paediatrics and Community Health Sciences, University of Calgary. All members of the Alberta FICare in Level II NICU study team contributed support and encouragement to conduct this study.
Ethics approval and consent to participate
This study was approved by the University of Calgary, Conjoint Health Research Ethics Board (CHREB ID 15-0067; covers Rockyview General Hospital, Peter Lougheed Centre, South Health Campus, Medicine Hat Regional Hospital, Chinook Regional Hospital, Red Deer Regional Hospital, and Queen Elizabeth II Hospital), University of Alberta, Health Research Ethics Board (Pro00060324; covers Royal Alexandra Hospital), and Covenant Health Research Ethics Board (ID 1762; covers Misericordia Community Hospital and Grey Nuns Community Hospital). This is version 2 of the protocol, approved on 9 June 2016. Minor changes to the ethics protocol were required when two graduate students were added to the study and required the addition of scales to assess breastfeeding patterns and self-efficacy. Mother participants provided written informed consent for themselves and their infant(s) to participate in the trial. Healthcare provider participants provided written informed consent to participate in qualitative interviews.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Canadian Institute for Health Information . Discharge Abstract Database/Hospital Morbidity Database: childbirth indicators by place of residence. Ottawa: Canadian Institute for Health Information; 2016.
-
- Tough S, Tofflemire K, Newburn-Cook C, Fraser-Lee N, Benzies K. Increased risks of pregnancy complications and adverse infant outcomes associated with assisted reproduction. Int Congr Ser. 2004;1271:376–9. doi: 10.1016/j.ics.2004.06.011. - DOI
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical