A series of N-of-1 trials to assess the therapeutic interchangeability of two enalapril formulations in the treatment of hypertension in Addis Ababa, Ethiopia: study protocol for a randomized controlled trial
- PMID: 29017595
- PMCID: PMC5634952
- DOI: 10.1186/s13063-017-2212-0
A series of N-of-1 trials to assess the therapeutic interchangeability of two enalapril formulations in the treatment of hypertension in Addis Ababa, Ethiopia: study protocol for a randomized controlled trial
Abstract
Background: Hypertension is one of the leading causes of morbidity and mortality in Ethiopia. Treatment usually involves lifelong medication use. Enalapril is a common drug for the treatment of hypertension in Ethiopia. However, the drug is expensive and, therefore, there is limited capacity for people to afford the treatment. Locally produced Enalapril is a cost-effective solution to treat the disease. However, as local medicines regulation does not include bioequivalence tests on locally produced drugs, physicians and patients need assurance about the effectiveness and safety of local generics. Evidence on therapeutic equivalence is needed on these untested local drugs.
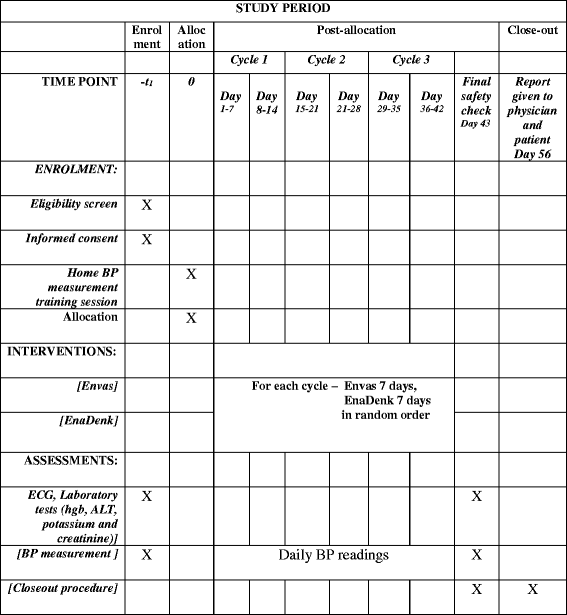
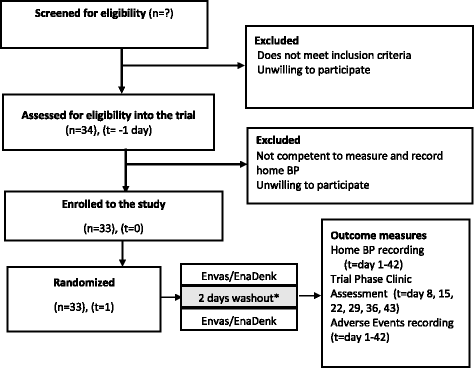
Methods: This is a hospital-based, randomized, partially blinded, three-cycle crossover trial in single patients, comparing a locally produced version of enalapril with enalapril imported from Europe. Patients involved in this trial are not blinded, as there is no local facility to produce relatively small numbers of placebos or encapsulated drugs. To ensure blinding of study investigators and data analysts, study medications are prepared by an independent pharmacy unit using opaque medication packaging. The importance of maintaining blinding is also part of patient pre-trial education. Each N-of-1 trial will consist of three successive 14-day treatment pairs, each pair comprising 7 days of 5-20 mg local and 7 days of 5-20 mg imported enalapril taken once daily in the morning. The primary outcome will be the average difference in systolic blood pressure as measured by home blood pressure measurements.
Discussion: The number of locally produced products, such as enalapril, being approved without proof of bioequivalence is dramatically increasing. By bridging the information gap on bioequivalence, the trial will give rigorous evidence on therapeutic equivalence of locally produced enalapril in the treatment of hypertension. If there is no difference, the hypothesized result, then patients can take the local medicine with confidence. This trial will also will determine whether aggregated N-of-1 studies are feasible to evaluate untested generic drugs in resource-limited countries where bioequivalence testing centers are unavailable.
Trial registration number: Australian and New Zealand Clinical Trial Registry, ID: ACTRN12616001088437p . Registered on 12 August 2016.
Keywords: Enalapril; Generic; Hypertension; N-of-1 trial; Randomized controlled trial; Therapeutic equivalence.
Conflict of interest statement
Ethics approvals and consent to participate
The study protocol and any accompanying materials are approved by both UQ and AAERC Institutional Review Boards. We have received AHRI ALERT Ethics Review Committee (AAERC) approval PO28/16 in Ethiopia and University of Queensland ethics approval 2016001230 in Brisbane.
A signed informed consent has to be obtained from each subject prior to participation (Patient Information Sheet and Informed Consent Form are attached as an Additional file 2).
This trial has been registered at the Australian New Zealand Clinical Trials Registry with the identifier ACTRN12616001088437p.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


 point estimate of the difference between groups,
point estimate of the difference between groups,  95% confidence intervals, *minimum clinically important difference (MCID)
95% confidence intervals, *minimum clinically important difference (MCID)References
-
- United Nation . World population prospect. 2015.
-
- Alwan A, Armstrong T, Cowan M, Riley L, World Health Organization . Noncommunicable diseases country profiles. Geneva: CH; 2011. p. 2011.
-
- Ethiopian Ministry of Health . Health and health-related indicators, 2008. 2008.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

